UK GUIDELINE FOR THE MANAGEMENT OF THE PATIENT WITH A FAILING KIDNEY TRANSPLANT
Chapters:
- Organisation of out-patient care
- Management of immunosuppression in failing allografts
- Surgical considerations
- Psychosocial issues
- Paediatrics
- Lifestyle and the failing kidney transplant
- Cardiovascular and metabolic risk management
- Complications of chronic kidney disease
- Management of the complications of long-term immunosuppression
- Patient involvement and options for renal replacement therapy
- Outcomes following return to dialysis or retransplantation
Introduction and need for the guideline
Despite significant advances in medical care over the last few decades, kidney transplants frequently do not function for the lifetime of recipients. 30 – 40 % of kidneys fail during the first 10 years following transplantation and around 3% of prevalent grafts fail annually.1 Patients whose transplants have failed currently constitute approximately 4% of the incident dialysis population and 16% of those on the transplant waiting list.1,2 As more kidney transplants are performed, it is inevitable that despite improvements in graft survival, the number of patients with failing grafts will increase. The cause of transplant failure is often multi-factorial, with chronic immune-mediated injury being a significant contributor.3-5
Recipients with a failing kidney transplant (RFKT) are complex and their management presents unique challenges.6-8 Ensuring optimal outcomes may include adjustment of immunosuppression9,10, consideration of co-morbidities11, diagnosis and management of allograft pathology and preparation for dialysis or retransplantation.12,13 It is well-recognised that there is significant morbidity and mortality associated with the time around return to dialysis, particularly in relation to cardiovascular disease and infection.14-18 However, the evidence to guide practice is limited and recommendations are usually based on personal experiences and available literature.
Many of the physiological changes that accompany the loss of graft function mimic those seen in progressive renal disease in the native kidneys. Most of these can be managed in a similar way to the non-transplant population and previous guidelines have made recommendations to this effect.19,20 However, there is evidence that kidney transplant recipients with poor graft function receive suboptimal care when compared to patients with progressive native kidney disease.2
As a result of these observations, several centres in the UK have established dedicated clinics for transplant patients with declining kidney function.21,22 Other units manage failing transplants in advanced kidney care clinics, or in standard transplant clinics with additional input from multidisciplinary teams as required.23 Due to increased co-morbidity and HLA sensitisation, preparation and waiting time for re-transplantation may be longer than for transplant-naïve recipients and should be born in mind. The gold standard of care for suitable patients is a pre-emptive, well-matched living donor transplant.24-27
The original guideline on the ‘Management of the patient with a failing kidney transplant’ was published in 2014. This revision aims to consider the emergence of recent evidence, an expansion of some aspects of care and the inclusion of new chapters, with specific relevance to kidney transplant recipients with poor graft function. We have added chapters addressing paediatric recipients, psychosocial considerations and the management of the complications of chronic kidney disease (CKD) in the context of a failing transplant. The previous chapters focused on cardiovascular and other risk factors have been broadened, with specific lifestyle recommendations included separately.
References
1 Organ and Tissue Donation and Transplantation Activity Report 2021/22.
2 UK Renal Registry 24th Annual Report.
3 Bohmig, G. A., Eskandary, F., Doberer, K. & Halloran, P. F. The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transpl Int 32, 775-788, doi:10.1111/tri.13436 (2019).
4 Sellares, J. et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12, 388-399, doi:10.1111/j.1600-6143.2011.03840.x (2012).
5 Halloran, P. F., Famulski, K. S. & Reeve, J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol 12, 534-548, doi:10.1038/nrneph.2016.85 (2016).
6 Davis, S. & Mohan, S. Managing Patients with Failing Kidney Allograft: Many Questions Remain. Clin J Am Soc Nephrol 17, 8, doi:10.2215/CJN.14620920 (2021).
7 Lubetzky, M. et al. The failing kidney allograft: A review and recommendations for the care and management of a complex group of patients. Am J Transplant 21, 2937-2949, doi:10.1111/ajt.16717 (2021).
8 Kidney Disease: Improving Global Outcomes (KDIGO). Controversies Conference on Challenges in Management of the Kidney Allograft: From Decline to Failure. March 2022., 2022).
9 Ryu, H. et al. Weaning Immunosuppressant in Patients with Failing Kidney Grafts and The Outcomes: A Single-Center Retrospective Cohort Study. Sci Rep 10, 6425, doi:10.1038/s41598-020-63266-3 (2020).
10 Knoll, G. et al. Immunosuppressant Medication Use in Patients with Kidney Allograft Failure: A Prospective Multicenter Canadian Cohort Study. J Am Soc Nephrol 33, 1182-1192, doi:10.1681/ASN.2021121642 (2022).
11 Rangaswami, J. et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant 34, 760-773, doi:10.1093/ndt/gfz053 (2019).
12 Fiorentino, M. et al. Management of patients with a failed kidney transplant: what should we do? Clin Kidney J 14, 98-106, doi:10.1093/ckj/sfaa094 (2021).
13 Moist, L. M. & Gill, J. S. Patient Management When Returning to Dialysis after a Failed Kidney Transplant. Clin J Am Soc Nephrol 16, 1423-1425, doi:10.2215/CJN.19731220 (2021).
14 Perl, J. et al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 27, 4464-4472, doi:10.1093/ndt/gfs386 (2012).
15 Bisigniano, L. et al. Reduced survival in patients who return to dialysis after kidney allograft failure. Clin Transplant 34, e14014, doi:10.1111/ctr.14014 (2020).
16 Kabani, R. et al. Risk of death following kidney allograft failure: a systematic review and meta-analysis of cohort studies. Nephrol Dial Transplant 29, 1778-1786, doi:10.1093/ndt/gfu205 (2014).
17 Rao, P. S. et al. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis 49, 294-300, doi:10.1053/j.ajkd.2006.11.022 (2007).
18 Kainz, A. et al. Waiting Time for Second Kidney Transplantation and Mortality. Clin J Am Soc Nephrol 17, 90-97, doi:10.2215/CJN.07620621 (2022).
19 Baker, R. J. M., P.B; Patel. R.K.; Stevens, K.K.; Palmer, N. . British Transplantation Society Clinical Practice Guideline (2017).
20 Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney trannsplant recipients. American Journal of Transplantation 9, 157 (2009).
21 Evans, R. D. R. et al. Assessment of a Dedicated Transplant Low Clearance Clinic and Patient Outcomes on Dialysis After Renal Allograft Loss at 2 UK Transplant Centers. Transplant Direct 4, e352, doi:10.1097/TXD.0000000000000788 (2018).
22 Arshad, A., Jackson-Spence, F. & Sharif, A. Development and evaluation of dedicated low clearance transplant clinics for patients with failing kidney transplants. J Ren Care 45, 51-58, doi:10.1111/jorc.12268 (2019).
23 Gittus, M., Bailey, P. & Griffin, S. V. in British Transplantation Society Annual Meeting (2023).
24 Johnston, O., Rose, C. L., Gill, J. S. & Gill, J. S. Risks and benefits of preemptive second kidney transplantation. Transplantation 95, 705-710, doi:10.1097/TP.0b013e31827a938f (2013).
25 Huml, A. M. & Schold, J. D. A Second Chance at Transplant First: Preemptive Repeat Kidney Transplantation. Kidney360 3, 11-13, doi:10.34067/KID.0007502021 (2022).
26 Vinson, A. J. et al. Disparities in Access to Preemptive Repeat Kidney Transplant: Still Missing the Mark? Kidney360 3, 144-152, doi:10.34067/KID.0003162021 (2022).
27 Girerd, S. et al. Preemptive second kidney transplantation is associated with better graft survival compared with non-preemptive second transplantation: a multicenter French 2000-2014 cohort study. Transpl Int 31, 408-423, doi:10.1111/tri.13105 (2018).
Process of writing and methodology
The Guideline has been written under the auspices of the BTS Guidelines and Standards Committee, in line with the British Transplant Society Guideline Development Policy1. An open call for contributory authors was issued in the Spring of 2021 and the writing group first met in June 2021. Contributing authors include a broad representation of the multi-disciplinary team involved in patient care, and transplant recipients.
The structure of the revised guideline is based on the previous edition, published in 2014. The overall writing group was divided into 10 sub-groups, each focussing on a separate chapter. The Centre for Evidence in Transplantation (CET) provided training to undertake a systematic literature search for each chapter. The content of each chapter was discussed by the wider group prior to collation for consultation. The identification of patient co-authors was facilitated by the Kidney Patient Involvement Network.
A review of the relevant literature was performed by the authors, and the recommendations contained within the guideline resulted from a collective decision reached following discussion. Consensus was obtained within the groups for selection of studies to cite. Where there is an overlap with an existing guideline, these are referenced to avoid duplication. For example, general management recommendations are described in the BTS Clinical Practice Guideline – Post-Operative Care of the Kidney Transplant Recipient. The focus of this guideline is on the failing graft and transitions to re-transplantation, dialysis, or conservative care.
This guideline is designed to be used by healthcare professionals working in the out-patient setting with kidney transplant recipients, their significant others and carers.
References
- https://bts.org.uk/wp-content/uploads/2021/05/BTS_Guideline_Development_Policy_2021.pdf
Contributing Authors
Professor Siân Griffin, Consultant Nephrologist, Cardiff and Vale UHB
Dr Elham Asgari, Consultant Nephrologist, Guys and St Thomas’ NHS Foundation Trust
Dr Adnan Sharif, Consultant Nephrologist, University Hospital Birmingham
Mr Alan Hancock, transplant recipient
Mr Alan Jones, transplant recipient
Dr Antonia Cronin, Consultant Nephrologist, Guys and St Thomas’ NHS Foundation Trust
Mr Andrew Barnett, Renal Social Worker
Ms Amanda Bevin, Renal Counsellor, East Kent Hospitals University HNS Foundation Trust
Mr Bruno Mafrici, Renal Dietician, Nottingham University Hospitals NHS Trust
Dr Caroline Dudreuilh, Consultant Nephrologist, Guys and St Thomas’ NHS Foundation Trust
Dr Charlotte Seneschall, Specialty Trainee, Imperial College London
Dr Clare McKeaveney, Psychologist, Queen’s University, Belfast
Mr Dominic Summers, Consultant Transplant Surgeon, Cambridge University Hospitals NHS Foundation Trust
Dr Gayathri Rajakaruna, Consultant Nephrologist, East and North Hertfordshire NHS Trust
Dr Gwendolyn Eich, Specialty Trainee, Great Ormond Street Hospital
Dr Hannah Burton, Consultant Nephrologist, Epsom and St Helier University Hospitals NHS Trust
Ms Hayley Wells, Renal Pharmacist, Guys and St Thomas’ NHS Foundation Trust
Dr Jelena Stojanovic, Consultant Paediatric Nephrologist, Great Ormond Street Hospital
Dr Joyce Popoola, Consultant Nephrologist, St George’s University Hospitals NHS Foundation Trust
Dr Konstantinos Koutroutsos, Consultant Nephrologist, Brighton and Sussex University Hospitals
Dr Lina Johansson, Renal Dietician, Imperial College London
Dr Maria Martinez, Renal Pharmacist, University Hospitals of Leicester NHS Trust
Dr Matt Gittens, Specialty trainee, Sheffield Teaching Hospital NHS Foundation Trust
Dr Matthew Robb, Principal Statistician, NHS Blood and Transplant
Dr Michelle Willicombe, Consultant Nephrologist, Imperial College London
Dr Mysore Phanish, Consultant Nephrologist, Epsom and St Helier University Hospitals NHS Trust
Mr Paul Maxted, transplant recipient
Dr Pippa Bailey, Associate Professor, University of Bristol and Honorary Consultant Nephrologist, Southmead Hospital, Bristol
Dr Rachel Davison, Consultant Nephrologist, Newcastle upon Tyne Hospitals NHS Foundation Trust
Dr Rachel Hilton, Consultant Nephrologist, Guys and St Thomas’ NHS Foundation Trust
Dr Richard Baker, Consultant Nephrologist, St James’s University Hospital, Leeds
Dr Rosa Montero, Consultant Nephrologist, St George’s University Hospitals NHS Foundation Trust
Dr Sarah Peacock, Director of Histocompatibility and Immunogenetics, Cambridge University Hospitals NHS Foundation Trust
Dr Sevda Hassan, Consultant Nephrologist, Barts Health NHS Trust
Ms Sharon Frame, Advanced Nurse Practitioner, Guys and St Thomas’ NHS Foundation Trust
Dr Sumoyee Basu, Specialty Trainee, Guys and St Thomas’ NHS Foundation Trust
Dr Suzanne Whitehead, Renal Psychologist, North Bristol NHS Trust
Dr Tina Thomson, Specialty Trainee, Imperial College London
Dr Zainab Arslan, Consultant Paediatric Nephrologist, Great Ormond Street Hospital
Conflicts of Interest
No conflicts of interest were declared.
Grading of Recommendations
These guidelines represent consensus opinion from clinical experts in the field of transplantation and patients in the United Kingdom. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system has been used to rate the strength of evidence and recommendations, consistent with other guidelines issued by the British Transplantation Society and other international, organisations. Explicit recommendations represent a balance between benefits and risks, burden, and cost.
The quality of evidence has been graded as:
A (high)
B (moderate)
C (low)
D (very low)
A High-quality evidence derived from consistent results from well-performed randomised controlled trials, or overwhelming evidence from another source (for example, well-executed observational trials with very strong effects).
B Moderate quality evidence from randomised trials that are compromised by flaws in conduct, consistency, indirectness, imprecise estimates, reporting bias or a combination of these limitations, or evidence from other studies with notable strength.
C Low quality evidence from observational studies, or controlled trials with significant limitations.
D Evidence is based on case studies or expert opinion.
Level 1 recommendation Strong recommendation, the benefits of an intervention clearly outweigh the risks for most, if not all patients.
Level 2 recommendation Weaker recommendation, where the risks and benefits are less certain or more closely balanced.
In many areas evidence is weak and based solely on expert opinion. On these occasions the authors felt guidance was appropriate to advise clinicians in day-to-day practice.
Abbreviations
ABPM Ambulatory Blood Pressure Monitoring
ACEi Angiotensin Converting Enzyme Inhibitor
ACR Albumin:Creatinine Ratio
AHUS Atypical Haemolytic Uraemic Syndrome
ARB Angiotensin Receptor Blocker
BTS British Transplantation Society
CAKUT Congenital Abnormalities of the Kidney and Urinary Tract
CAPD Continuous Ambulatory Peritoneal Dialysis
CCB Calcium Channel Blocker
CKD Chronic Kidney Disease
CNI Calcineurin Inhibitor
cRF Calculated Reaction Frequency
DSA Donor Specific Antibody
ESKD End-Stage Kidney Disease
FSGS Focal Segmental Glomerulosclerosis
GFR Glomerular Filtration Rate
HD Haemodialysis
HSP Highly Sensitised Patient
ISPD International Society for Peritoneal Dialysis
KDIGO Kidney Disease: Improving Global Outcomes
KTR Kidney Transplant Recipient
MDT Multi-Disciplinary Team
MMF Mycophenolate Mofetil
MCGN Mesangiocapillary glomerulonephritis
NHSBT NHS Blood and Transplant
ODT Organ Donation and Transplantation
PCR Protein:Creatinine Ratio
PD Peritoneal Dialysis
PRA Panel Reactive Antibody
RFKT Recipient with a Failing Kidney Transplant
RRT Renal Replacement Therapy
TRAS Transplant Renal Artery Stenosis
UKKA UK Kidney Association
VUR Vesico-ureteric reflux
YAW Young Adult Worker
Definitions
Failing Kidney transplant
There is no agreed consensus on the definition of a failing kidney transplant. For the purpose of this guideline, we have included two groups:
- Those with stable but low baseline kidney function, for example eGFR <20 ml/minute/1.73 m2. Although transition to re-transplantation, dialysis or conservative care may not be planned for these recipients, they would likely benefit from the multi-disciplinary approach of an advanced kidney care clinic.
- Those with an irreversible and progressive decline in kidney function and an anticipated transplant survival of <12 months. This second group will in addition need support for their transition to their next mode of renal replacement therapy.
Advanced kidney care clinic
Advanced kidney care clinics have evolved to provide multi-disciplinary input for patients with advanced kidney disease and low kidney function. The composition of the MDT varies between centres, but typically includes a nephrologist, specialist renal nurses, dietitian, psychologist and social worker.
Core support group
Anyone that the patient views as important in their life and they would like involved in shared decision-making. It may include family, friends, carers, neighbours, work colleagues, support workers etc.
Disclaimer
This document provides a guide to best practice, which inevitably evolves over time. All clinicians involved in these aspects of transplantation need to undertake clinical care on an individualised basis and keep up to date with changes in the practice of clinical medicine.
These guidelines represent the collective opinions of experts in the field and do not have the force of law. They contain information/guidance for use by practitioners as a best practice tool. It follows that the guidelines should be interpreted in the spirit rather than the letter of their contents. The opinions presented are subject to change and should not be used in isolation to define the management of any individual patient.
The guidelines are not designed to be prescriptive, nor to define a standard of care. The British Transplantation Society cannot attest to the accuracy, completeness or currency of the opinions contained herein and does not accept responsibility or liability for any loss or damage caused to any practitioner or any third party as a result of any reliance being placed on the guidelines or as a result of any inaccurate or misleading opinion contained in the guidelines.
1. ORGANISATION OF OUT-PATIENT CARE
Statements of Recommendation
We suggest that:
- Patients with failing grafts have ready access to the low-clearance multi-disciplinary team. (2C)
- Joint transplant/advanced kidney care be initiated at least 12 months before the anticipated need for dialysis or retransplantation, or when graft eGFR falls below 20ml/minute/ 1.73 m2. (2C)
- Where appropriate, retransplantation be undertaken when the eGFR of the recipient with a failing kidney transplant has fallen to 10 – 15 ml/minute/1.73 m2. (2C)
- Given their increased morbidity, particular attention should be paid to the attainment of cardiovascular and other targets. (2C)
Rationale
In most centres in the UK, recipients with failing kidney transplants (RFKTs) continue to be managed in general transplant clinics with additional specialist input as required1. There is evidence from the Renal Registry that kidney transplant recipients with poor graft function receive inferior care compared to patients with native kidney disease and are less likely to achieve recommended targets for control of hypertension and metabolic complications2. In addition, poor kidney transplant function is associated with an increased risk of death3, a risk largely attributable to higher rates of cardiovascular disease and infection. Although particularly high at the time of return to dialysis, the increased risk persists4-8.
These observations have given impetus to the establishment of dedicated advanced kidney care transplant clinics, with the aim of improving outcomes for this patient group, but their benefits remain unproven9,10. The model of care will vary depending on the number of patients, geography, and availability of resources, but regardless of this, RFKTs should have ready access to the guidance and advice of the multi-disciplinary team. In line with pre-dialysis patients, joint transplant/advanced kidney care should be initiated at least 12 months before the anticipated need for dialysis or re-listing for transplantation11,12. The optimal timing of re-transplantation will depend on several factors including the rate of change of kidney function and symptom burden, and in the case of living donor transplantation, donor, and recipient convenience. In the absence of indications for delay (for example, following treatment for malignancy), preparations for re-transplantation should be completed by the time the eGFR has fallen to 10 – 15 ml/minute/1.73 m2.
References
1 Gittus, M., Bailey, P. & Griffin, S. V. in British Transplantation Society Annual Meeting (2023).
2 UK Renal Registry 24th Annual Report.
3 Kabani, R. et al. Risk of death following kidney allograft failure: a systematic review and meta-analysis of cohort studies. Nephrol Dial Transplant 29, 1778-1786, doi:10.1093/ndt/gfu205 (2014).
4 Rao, P. S. et al. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis 49, 294-300, doi:10.1053/j.ajkd.2006.11.022 (2007).
5 Perl, J. et al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 27, 4464-4472, doi:10.1093/ndt/gfs386 (2012).
6 Bisigniano, L. et al. Reduced survival in patients who return to dialysis after kidney allograft failure. Clin Transplant 34, e14014, doi:10.1111/ctr.14014 (2020).
7 Gill, J. S., Abichandani, R., Kausz, A. T. & Pereira, B. J. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int 62, 1875-1883, doi:10.1046/j.1523-1755.2002.00640.x (2002).
8 Ojo, A. O. et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate Analyses from the United States Renal Data System. Transplantation 66, 9 (1998).
9 Evans, R. D. R. et al. Assessment of a Dedicated Transplant Low Clearance Clinic and Patient Outcomes on Dialysis After Renal Allograft Loss at 2 UK Transplant Centers. Transplant Direct 4, e352, doi:10.1097/TXD.0000000000000788 (2018).
10 Arshad, A., Jackson-Spence, F. & Sharif, A. Development and evaluation of dedicated low clearance transplant clinics for patients with failing kidney transplants. J Ren Care 45, 51-58, doi:10.1111/jorc.12268 (2019).
11 Lubetzky, M. et al. The failing kidney allograft: A review and recommendations for the care and management of a complex group of patients. Am J Transplant 21, 2937-2949, doi:10.1111/ajt.16717 (2021).
12 Fiorentino, M. et al. Management of patients with a failed kidney transplant: what should we do? Clin Kidney J 14, 98-106, doi:10.1093/ckj/sfaa094 (2021).
2. MANAGEMENT OF IMMUNOSUPPRESSION IN FAILING ALLOGRAFTS
Statements of Recommendation
We recommend that:
- Where the allograft is failing (eGFR < 20ml/minute/1.73m2 and declining) AND there is a likelihood of future retransplantation, immunosuppression (IS) is maintained to preserve residual kidney function and avoid allograft sensitisation. (1C)
- Where the allograft has failed (return to dialysis) AND there is a likelihood of future retransplantation there is reduction but not the withdrawal of IS to minimise IS-related complications such as infection and malignancy while avoiding allograft sensitisation. (1C)
- Where the allograft has failed (return to dialysis) and there is NO likelihood of future retransplantation, there is tapering and ultimately complete withdrawal of IS to minimise IS-related complications. (1C)
- For patients where there is a likelihood of retransplantation, there is 3 monthly monitoring of HLA antibodies. (1D)
- Where IS has been reduced or withdrawn there is surveillance for graft intolerance syndrome. (1D)
- In the event of graft intolerance syndrome following the withdrawal of IS, steroid therapy is recommenced. (1C)
We suggest that:
- Where the allograft is failing (eGFR < 20ml/minute/1.73m2 and declining) and there is no likelihood of future retransplantation we advise gradual reduction (but not complete withdrawal) of IS to minimise IS-related complications while preserving residual kidney function. (ungraded)
- If acute inflammation does not resolve following steroid treatment for graft intolerance syndrome, transplant nephrectomy may be considered. (2D)
- Reduction of IS after graft nephrectomy needs consideration of the time since transplant, retention of residual donor tissue and prospects for retransplantation. (2D)
Recommendations for future research:
- Randomised controlled trial of IS withdrawal and/or nephrectomy.
- Is monitoring of IS levels during tapering required?
- Age stratification for IS intensity with consideration of the presence of immunosenescence in older recipients.
- Potential role of novel immunosuppressants, for example, belatacept-based regimens, to prolong the function of a failing allograft and reduce the development of de novo donor-specific antibodies.
Rationale
2.1 Introduction
Allograft failure is an increasingly prevalent cause of End Stage Kidney Disease requiring the return to dialysis or retransplantation. This is largely due to the growth in the absolute number of kidney transplants rather than an increased rate of transplant failures. In the UK, on 31/12/2020, 16% of the 38,895 prevalent transplant recipients had a GFR <30 ml/minute/1.73m2 indicating a poorly functioning or failing renal allograft (24th Renal Registry Report).1
A significant proportion of recipients with failed kidney transplants (RFKT) will be relisted for transplantation and comprise 16% of the active and suspended kidney transplant waiting pool (NHSBT data, unpublished).
The majority (up to 60%) of patients listed for repeat transplantation are sensitised (detailed in section 11). Many of these patients are highly sensitised with a concomitantly reduced probability of receiving an offer of a compatible transplant. Recent studies have shown that the development of HLA sensitisation after transplant failure occurs most often after returning to the transplant waiting list, when awaiting repeat kidney transplantation. This usually coincides with immunosuppression (IS) tapering or withdrawal2 and is independent of graft nephrectomy.3,4 It is important to consider the timescale for repeat transplantation, for example, imminent live donor transplantation (in which case continuing IS is usually justified) or return to the deceased donor kidney transplant waiting list where waiting time may vary from months to years (in which case continuing IS will require careful consideration of risks and benefits for the individual patient). This is particularly relevant for paediatric recipients and young adults who are likely to require retransplantation within their lifetime. Clinical practice varies amongst centres; this has been described in two different US surveys.5,6 Ultimately an individualised approach is required where the risk of ongoing IS exposure is balanced against the risks of sensitisation7.
2.2 Patient considered for retransplantation
Ongoing IS preserves residual graft function, prevents graft intolerance syndrome, and helps avoid sensitisation but has potential complications such as infection and malignancy. However, this needs to be balanced against the risk of HLA sensitisation.
2.2.1 Risk of alloimmunisation versus continued immunosuppression
The most compelling argument to continue IS is to prevent a rise in HLA-specific antibody levels, which may reduce the likelihood of retransplantation and lead to increased waiting time. Sensitisation frequently develops following IS withdrawal. Emerging evidence suggests early withdrawal of IS may be an independent risk factor for the development of HLA sensitisation. In a cohort of 119 recipients who had only low levels of sensitisation prior to transplantation, Augustine et al found that the percentage of highly sensitised patients increased from 21% to 68% in the group in whom IS was weaned. In the cohort in whom IS was maintained, there was minimal change in sensitisation3. Rao et al demonstrated that a rapid withdrawal of IS was associated with an increased Class I panel reactive antibody (PRA), trended towards the development of Donor Specific Antibodies (DSA), and increased Class II PRA when compared with a gradual reduction in IS.8 Casey et al reported a lower rate of sensitisation at transplant evaluation in recipients with prolonged IS versus early withdrawal.9
Transplant nephrectomy was formerly considered an option to allow withdrawal of IS without provoking sensitisation. This approach has not been supported by recent studies. Serial Luminex analysis suggests that in recipients with DSA, the level of these may rise following graft nephrectomy independent of IS withdrawal.4 It has been postulated that this may be due to the graft having acted as an ‘antibody sink’ or the pro-inflammatory effect of the surgical procedure.10 Nimmo et al compared changes in calculated reaction frequency (cRF) during weaning of IS in patients who had or had not undergone nephrectomy. In 42 recipients who had undergone nephrectomy, cRF increased from 31% prior to IS reduction, 69% after IS reduction to 89% post-IS cessation. This compared to changes from 13% to 40% to 62% in the same IS weaning categories for 17 patients who had a failed graft in situ.11This equated to a reduction in the relative chance of re-transplantation from 54% to 46% at 5 years for the patients with graft in situ and from 54% to 42% at 5 years for patients who had undergone nephrectomy. Similarly in a study of 91 nephrectomies, patients with a PRA < 20% had a significant PRA increase post nephrectomy while patients with PRA > 80% exhibited a significant but small decrease in PRA.12
Two recent retrospective studies13, 14 support the continuation of CNI for more than 3 and 6 months respectively after graft failure to reduce the rate of sensitisation. Absence of CNI at 6 months after graft failure was significantly associated with calculated PRA > 75% (OR 4.8, CI 95% 1.5–15.0, p = 0.006) and de novo DSA development (OR 23.2, CI 95% 5.3–100.6, p < 0.001). The development of de novo DSAs after cessation of CNI after 3 months (n = 63/90 [70.0%]) was significantly more frequent than during CNI treatment (n = 18/52 [34.6%], P = 0.01).
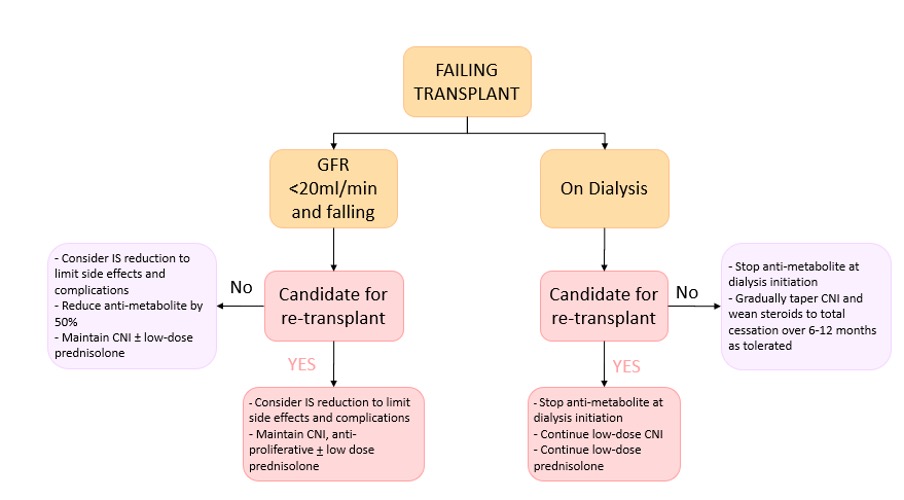
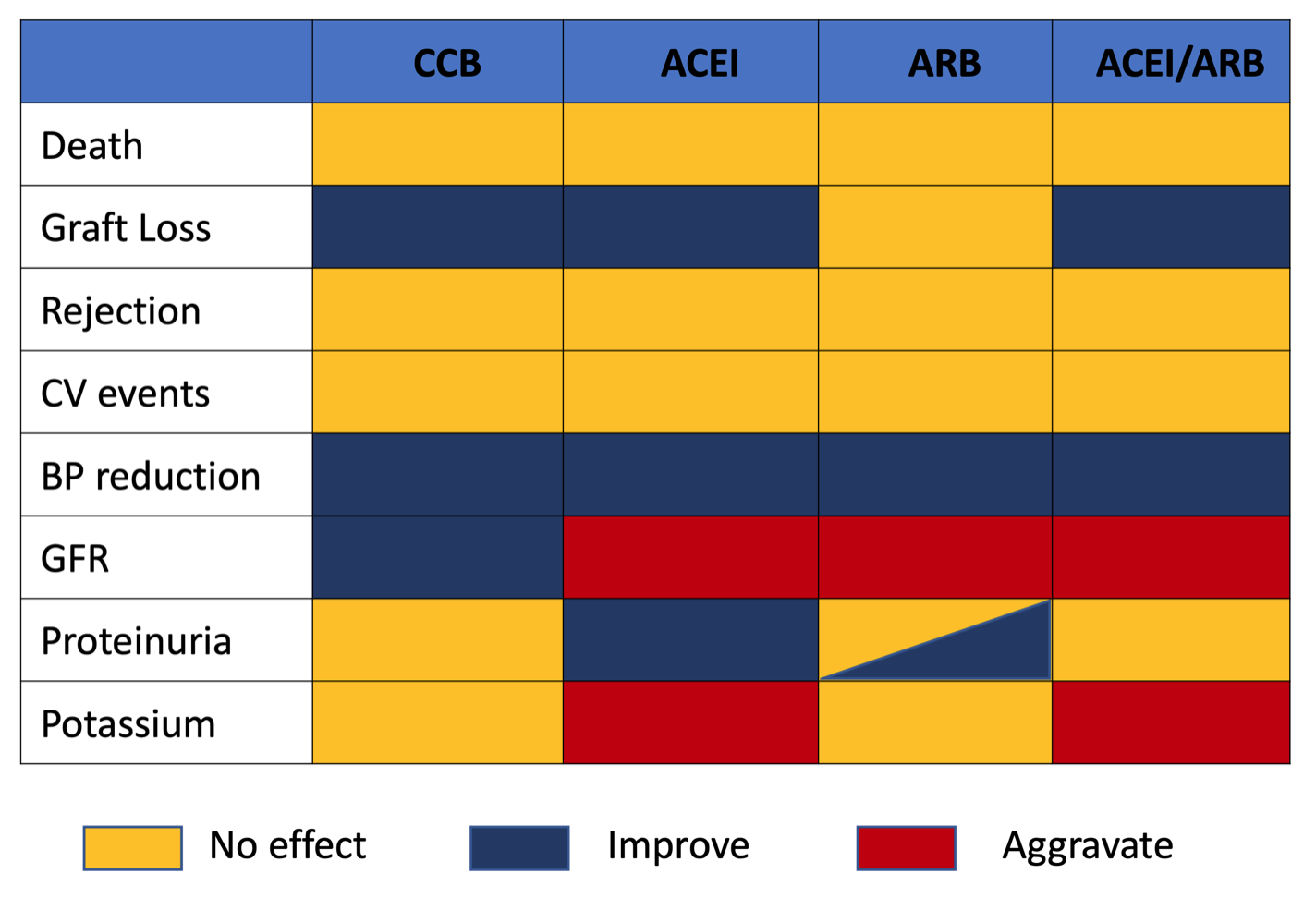
A reasonable approach (Figure 1) is to minimise the risk of immunological sensitisation by continuing IS therapy if there is a likelihood of future transplantation. If a longer period is anticipated before repeat transplantation, the risk: benefit ratio may favour gradual withdrawal of IS and should be considered on an individual basis.
There are specific situations in which recipients may require a rapid decrease in IS, notably BK virus nephropathy15or certain forms of malignancy.
Figure 1: Summary of recommendations for immunosuppression management in the failing and failed transplant
2.2.2 HLA-specific antibody screening
Recipients with a failing kidney transplant are at high risk of developing de novo sensitisation. This is particularly the case after starting dialysis or re-listing for transplantation, when IS is commonly changed, reduced, or withdrawn.
Antibody screening after graft failure and on return to the transplant waiting list should be undertaken according to the BTS/BSHI Guidelines for the Detection and Characterisation of Clinically Relevant Antibodies in Solid Organ Transplantation [2015] (https://bts.org.uk/wp-content/uploads/2016/09/06_BTS_BSHI_Antibodies-1.pdf) and Eurotransplant guidance.16
For potential kidney recipients on the waiting list, routine HLA-specific antibody screening must be performed every three months. The clinical team should notify the histocompatibility laboratory of any potential immunising events (blood transfusion, transplantation, pregnancy, graft removal, infections or vaccination) and send serum samples for HLA-specific antibody screening two to four weeks after these events.16 The laboratory should also be informed if the patient has received treatment with monoclonal antibodies that interfere with assays (for example rituximab or daratumumab).
2.3 Patient not to be considered for relisting
If retransplantation will never be an option, then concerns about sensitisation do not apply. The management aim for these patients is to reduce the burden of unnecessary IS when no longer required, however, there is at yet no prospective trial data to provide specific guidance.
The increased burden of morbidity and mortality from prolonged IS is clearly recognised. This includes increased rates of malignancy, infection16-19, dyslipidemia, post-transplant diabetes mellitus (PTDM) and cardiovascular disease. A recent study of 131 failing transplant recipients20 demonstrated that maintaining IS 6 months after allograft failure (prednisolone >10mg or more than two IS drugs) elevated the risk of all-cause mortality approximately three-fold compared to patients on no IS or prednisolone <10mg. Similarly, Friest et al found significantly higher infection rates in those on prolonged corticosteroids (>3 months) versus early cessation in multivariate analysis of a retrospective cohort, but no difference in the development of DSA.
In contrast, the risks of withdrawal of IS include:
- precipitation of graft intolerance syndrome and the potential need for transplant nephrectomy3,21
- loss of residual renal function10
- secondary adrenal insufficiency following cessation of corticosteroids22
- erythropoietin resistance related to the chronic inflammatory stimulus of the graft (although this is balanced by the myelotoxicity associated with some agents)
There is no clear evidence to guide the optimal management of IS in patients with late graft failure. A staged approach to IS withdrawal is usually considered appropriate. This is practised by most centres, with discontinuation of anti-metabolites, tapering CNI over several weeks and prednisolone over 3- 6 months.15, 23 Anti-metabolites are more likely to contribute to myelosuppression than CNIs and are less effective at preventing sensitisation.15
It is recommended that steroids are the last component to be withdrawn. To minimise the risk of iatrogenic hypoadrenalism, prednisolone should not be withdrawn faster than 1 mg per month once the dose is below 5 mg daily. In the event of clinical manifestations of adrenal insufficiency such as hypotension or hypoglycaemia, it is appropriate to reintroduce steroids at the previous dose and to attempt a slower steroid taper.
2.4 Graft Intolerance Syndrome
Graft intolerance syndrome, due to a severe acute rejection with manifestations such as pain over the graft, fever, haematuria, raised inflammatory markers and thrombocytopenia is a recognised complication of IS withdrawal and is described in up to 50% of patients if the withdrawal has been rapid.24 Presentation may also be more subtle with non-specific symptoms. Following a retrospective review of 149 recipients, Delgado et al25 reported that most graft intolerance episodes occurred within 6 months and virtually all presented within 2 years of graft failure. Consequently, monitoring should be most vigilant for the first year following the transition to dialysis. In this event, steroid therapy should be immediately reinstituted according to local practice (employed strategies include 20mg or 1mg/kg prednisolone daily or pulsed IV methylprednisolone 500mg) followed by transplant nephrectomy when the acute inflammation has settled. Woodside et al found that 38% of febrile hospitalised RFKT who had been weaned of IS before admission had documented infection, inferring the remainder had graft intolerance syndrome in contrast to 88% of patients maintained on IS having documented infection (P<0.001). A multivariate analysis of a retrospective cohort indicated that older donor age, shorter graft survival and higher number of rejection episodes were predictive of graft intolerance syndrome necessitating nephrectomy.26
2.5 Management of immunosuppression following transplant nephrectomy
There is no clear evidence for the management of IS following transplant nephrectomy, but we suggest that the time since transplant is considered.
Immediate: if there has been complete removal of all donor tissue there is no need for ongoing immunosuppression.
Early (within the first few days): transplantation should be considered a significant sensitising event, with HLA antibodies developing even in patients who have subsequently undergone nephrectomy within 24 hours.27 We, therefore, suggest gradual withdrawal of IS rather than abrupt cessation.
Late: substantial amounts of donor tissue may remain. If re-transplantation is planned, IS withdrawal should be gradual and at a rate similar to that of recipients who have returned to dialysis with a transplant in situ. If re-transplantation is not planned, IS withdrawal can be more rapid, with the caveat above.
References
- UK renal registry 24th annual report. https://ukkidney.org/sites/renal.org/files/publication/file-attachments/24th_UKRR_ANNUAL_REPORT_BOOK%20version%203_0.pdf
- Scornik JC, Kriesche HM. Human leukocyte antigen sensitization after transplant loss: timing of antibody detection and implications for prevention. Human Immunology 2011: 72: 398–401.
- Augustine JJ, Woodside KJ, Padiyar A, Sanchez EQ, Hricik DE, Schulak JA: Independent of nephrectomy, weaning immunosuppression leads to late sensitization after kidney transplant failure. Transplantation 94:738–743, 2012
- Del Bello A, Congy-Jolivet N, Sallusto F, Guilbeau-Frugier C, Cardeau-Desangles I, Fort M, Esposito L, Guitard J, Cointault O, Lavayssie` re L, Nogier MB, Blancher A, Rostaing L, Kamar N: Donor-specific antibodies after ceasing immunosuppressive therapy, with or without an allograft nephrectomy. Clin J Am Soc Nephrol 7: 1310–1319, 2012
- Bayliss GP, Gohh RY, Morrissey PE, Rodrigue JR, Mandelbrot DA. Immunosuppression after renal allograft failure: a survey of US practices. Clin Transplant. 2013;27(6):895-900.
- Alhamad T, Lubetzky M, Lentine KL, et al. Kidney recipients with allograft failure, transition of kidney care (KRAFT): A survey of contemporary practices of transplant providers. Am J Transplant. 2021.
- Lea‐Henry, T. and Chacko, B., 2018. Management considerations in the failing renal allograft. Nephrology, 23(1), pp.12-19.
- Rao PS, Schaubel DE, Jia X et al. Survival on dialysis postkidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis 2007; 49: 294–300
- Casey MJ, Wen X, Kayler LK et al. Prolonged immunosuppression preserves nonsensitization status after kidney transplant failure. Transplantation 2014; 98: 306–311
- Davis S, Mohan S. Managing Patients with Failing Kidney Allograft: Many Questions Remain. Clin J Am Soc Nephrol. 2021 Mar 10:CJN.14620920. doi: 10.2215/CJN.14620920. Epub ahead of print. PMID: 33692118.
- Nimmo AMSA, McIntyre S, Turner DM, Henderson LK, Battle RK. The Impact of Withdrawal of Maintenance Immunosuppression and Graft Nephrectomy on HLA Sensitization and Calculated Chance of Future Transplant. Transplant Direct. 2018 Nov 23;4(12):e409. doi: 10.1097/TXD.0000000000000848. PMID: 30584590; PMCID: PMC6283087.
- Khakhar AK, Shahinian VB, House AA et al. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcomes. Transplant Proc 2003; 35: 862–863
- Freist, Marine, Dominique Bertrand, Elodie Bailly, Céline Lambert, Paul Olivier Rouzaire, Richard Lemal, Julien Aniort, Matthias Büchler, Anne Elisabeth Heng, and Cyril Garrouste. “Management of Immunosuppression After Kidney Transplant Failure: Effect on Patient Sensitization.” In Transplantation Proceedings, vol. 53, no. 3, pp. 962-969. Elsevier, 2021.
- López del Moral Cuesta, Covadonga, Sandra Guiral Foz, David Gómez Pereda, José Luis Pérez Canga, Marina de Cos Gómez, Jaime Mazón Ruiz, Ana García Santiago et al. “Immunosuppression with Calcineurin Inhibitor after Renal Transplant Failure Inhibits Allosensitization.” Biomedicines 8, no. 4 (2020): 72.
- Michelle Lubetzky , Ekamol Tantisattamo, Miklos Z Molnar, Krista L Lentine, Arpita Basu, Ronald F Parsons, Kenneth J Woodside, Martha Pavlakis, Christopher D Blosser, Neeraj Singh, Beatrice P Concepcion, Deborah Adey, Gaurav Gupta, Arman Faravardeh, Edward Kraus, Song Ong, Leonardo V Riella, John Friedewald, Alex Wiseman, Amtul Aala, Darshana M Dadhania, Tarek Alhamad. The failing kidney allograft: A review and recommendations for the care and management of a complex group of patients. Am J Transplant. 2021 Sep;21(9):2937-2949. doi: 10.1111/ajt.16717.
- Eurotransplant Manual© – version 4.5; November 20, 2018. https://www.eurotransplant.org/wp-content/uploads/2020/01/H10-Histocompatibility.pdf
- Gregoor PJ, Kramer P, Weimar W, van Saase JL. Infections after renal allograft failure in patients with or without low-dose maintenance immunosuppression. Transplantation. 1997;63(10):1528- 1530.
- Johnston O, Zalunardo N, Rose C, Gill JS. Prevention of sepsis during the transition to dialysis may improve the survival of transplant failure patients. J Am Soc Nephrol 2007; 18:1331-1337
- van Leeuwen MT, Webster AC, McCredie MR, et al. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ. 2010;340:c570.
- Ryu, Hyunjin, Yong Chul Kim, Jong Joo Moon, Eun Young Song, Sang-il Min, Jongwon Ha, Kwon Wook Joo, Yon Su Kim, Curie Ahn, and Hajeong Lee. “Weaning immunosuppressant in patients with failing Kidney Grafts and the outcomes: A Single-center Retrospective cohort Study.” Scientific reports 10, no. 1 (2020): 1-9.
- Madore F, Hébert MJ, Leblanc M, Girard R, Bastien E, Morin M, Beaudry C, Boucher A, Dandavino R. Determinants of late allograft nephrectomy. Clin Nephrol 1995 Nov;44(5):284-9.
- Verresen L, Vanrenterghem Y, Waer M, Hauglustaine D, Michielsen P. Corticosteroid withdrawal syndrome in dialysis patients. Nephrol Dial Transplant 1988; 3: 476-477
- Elmahi N, Csongradi E, Kokko K, Lewin JR, Davison J, Fulop T. Residual renal function in peritoneal dialysis with failed allograft and minimal immunosuppression. World J Transplant 2013 June 24; 3(2):26-29.
- Pham, P.T. and Pham, P.C., 2011, May. Immunosuppressive management of dialysis patients with recently failed transplants. In Seminars in dialysis (Vol. 24, No. 3, pp. 307-313). Oxford, UK: Blackwell Publishing Ltd.
- Delgado, P., Diaz, F., Gonzalez, A., Sanchez, E., Gutierrez, P., Hernandez, D., Torres, A. and Lorenzo, V., 2005. Intolerance syndrome in failed renal allografts: incidence and efficacy of percutaneous embolization. American journal of kidney diseases, 46(2), pp.339-344.
- Bunthof KLW, Verhoeks CM, van den Brand JAJG, Hilbrands LB. Graft intolerance syndrome requiring graft nephrectomy after late kidney graft failure: can it be predicted? A retrospective cohort study. Transpl Int. 2018 Feb;31(2):220-229. doi: 10.1111/tri.13088. Epub 2017 Nov 16. PMID: 29082567.
- Gaetano Lucisano, Paul Brookes, Eva Santos-Nunez, Nicola Firmin, Nicola Gunby, Sevda Hassan, Alexander Gueret-Wardle, Paul Herbert, Vassilios Papalois, Michelle Willicombe & David Taube. Allosensitization after transplant failure: the role of graft nephrectomy and immunosuppression – a retrospective study. Transplant International 2019; 32:949-959
3. SURGICAL CONSIDERATIONS
Statements of Recommendation
We suggest that:
- Graft nephrectomy should be considered on a case-by-case basis. (ungraded)
- Asymptomatic graft nephrectomy is not recommended outside of a clinical trial. (ungraded)
- Percutaneous embolization may be considered for high-risk patients with graft-intolerance syndrome. (2D)
- Patients with failing transplants should be referred early for vascular access to ensure they optimise their likelihood of restarting dialysis using a fistula or graft, or peritoneal dialysis as appropriate. (2D)
Rationale
3.1 Introduction
There is considerable international variation in the incidence of transplant nephrectomy following graft failure, with reported rates in a recent review ranging between 20-80% in patients undergoing retransplantation1. There is no published registry data from the UK, although a recent unpublished survey suggests that rates are substantially lower. There is little evidence to explain this variation in practice. This chapter presents possible indications and a description of available techniques, along with evidence for vascular access strategies prior to restarting dialysis.
3.2 Graft nephrectomy
3.2.1 Indications
Graft nephrectomy is a major operation associated with a significant risk of morbidity and mortality. In addition, there are other potential disadvantages to graft nephrectomy such as the loss of residual allograft endocrine function and urine output.
There are broadly accepted indications for graft nephrectomy, including acute peri-transplant vascular thrombosis, risk of graft rupture, graft malignancy, recurrent sepsis, graft intolerance syndrome, requirement for immunosuppression withdrawal and to create space for further transplants. Graft rupture is an uncommon early complication of renal transplantation, typically associated with severe acute rejection2. Graft malignancy, either de-novo or donor-derived is very uncommon with renal cell carcinoma the most commonly reported malignancy3,4. Chronic intractable pyelonephritis has been widely reported as an indication for graft nephrectomy. Nephrectomy following graft loss due to BK nephropathy, is not clearly indicated. Graft intolerance syndrome is a chronic inflammatory state associated with the failed transplant and can be characterised by fever, graft tenderness, haematuria, malaise and refractory anaemia5. One series suggests that the incidence of graft intolerance syndrome in patients returning to dialysis is as high as 30% 6.
Graft nephrectomy in the context of an asymptomatic graft is controversial. A large US-based retrospective cohort study suggested that there was a 32% lower adjusted relative risk of mortality in patients who underwent graft nephrectomy following a return to dialysis7, attributed to a reduction in the risk of chronic inflammation. However, the study could not clearly identify the clinical decisions leading to nephrectomy and so is at risk of substantial bias8.
The relationship between graft nephrectomy and HLA sensitisation is also controversial with several cohort studies demonstrating conflicting results. A recent review summarised 13 studies investigating the impact of graft nephrectomy on panel reactive antibody levels (PRA)1. Seven of the 13 studies demonstrated significantly higher levels of PRA in patients who had undergone nephrectomy compared to those who did not. However, other small studies suggest that this is related to the underlying indications for nephrectomy and management of immunosuppression, rather than nephrectomy per se. This includes one UK-based single centre cohort study of relisted transplant patients and showed a 3-fold increased risk of developing HLA-specific antibodies, but after controlling for immunosuppression cessation, this effect disappeared9.
3.2.2 Technique and prognosis
Most adult transplants in the UK are placed extraperitoneally10. During the early peri-transplant period, up to 6 weeks following the transplant, it is usually possible to mobilise the kidney and vessels from the peritoneum and remove most donor tissue including vessels and the ureter. Typically, a small patch of the donor’s vessels is retained in the recipient to avoid the need for patch repair of the recipient’s vessel, although native vessels (for example a saphenous or inferior epigastric patch) may be used to ensure complete removal of donor tissue.
After the immediate perioperative period, the capsule and peritoneum become fused, and there are two described techniques to perform the nephrectomy – intra- and extra-capsular. Due to the adherence of the kidney capsule to recipient tissues, the most technically straightforward technique is intracapsular – separating the kidney parenchyma from the capsule, and dividing the vessels and ureter in the hilum of the kidney11. The disadvantage of this technique is that significant quantities of donor tissue may remain, and it does not provide an oncologically clear margin in the case of donor kidney neoplasia. An alternative is an extracapsular approach in which the entire kidney is removed, leaving only donor blood vessels as patches on the recipient’s vessels.
Two small case series describe results comparing the two techniques. Touma et al excluded patients with early graft loss and described that an intracapsular approach results in shorter operative times and reduced blood loss, with no additional risk of sensitisation, compared to an extra-capsular technique12. Vavallo et al included patients with early graft loss and described no difference in peri-operative complications, although the extra-capsular approach was associated with prolonged hospital stay compared to the intracapsular approach. This may reflect the acute nature of early nephrectomy.
Reported morbidity and mortality associated with nephrectomy varies widely across case series, era and with indication. A recent review describes mortality rates from small cohort studies ranging between 0-11%, with post-operative infection, bleeding, bowel ischaemia and intravascular coagulopathy cited as causes of death. Complication rates vary between 5 and 48% in the reported series, with post-operative bleeding and haematoma formation being the most common 1.
Renal artery embolization has been reported as a potential alternative to nephrectomy in the symptomatic failed transplant. A recent review described the outcomes of 189 patients who underwent percutaneous embolization compared to 2232 patients who underwent nephrectomy for a range of indications, including graft intolerance syndrome, acute and chronic rejection. The reported mortality associated with embolization was 0.1% compared to 4% in the nephrectomy group, although 20% of patients needed post-embolisation nephrectomy13. There was no information about the risk of development of anti-HLA antibodies and these studies were at risk of significant bias due to variation in recipient selection and nephrectomy indication.
3.3 Dialysis Access
Return to dialysis following failed transplantation is likely to be more complex than at first presentation with renal disease. This is multifactorial, due to the history of abdominal surgery and the likelihood of previous vascular access, including fistula formation. Haemodialysis established via a native arterio-venous fistula is associated with improved longevity in the general dialysis population when compared to graft and central venous catheters14,15. However, a national report from the US suggests that 65% of patients restarting haemodialysis after a failed kidney transplant do so via a central venous catheter16, and data from two centres in the UK suggested that around 50% of patients restarted haemodialysis via a central venous catheter17. We recommend an early referral to dialysis access services to maximise the chance of starting dialysis via a fistula.
The same series demonstrated that around 15% of patients restarted peritoneal dialysis, and a large Canadian cohort study with over 2000 patients did not identify any difference in mortality between patients treated with peritoneal dialysis or haemodialysis following graft loss18. Early surgical assessment is recommended to ensure peritoneal dialysis is available when required.
References
- Ghyselen L, Naesens M. Indications, risks and impact of failed allograft nephrectomy. Transplant Rev. 2019;33(1):48-54. doi:https://doi.org/10.1016/j.trre.2018.08.001
- Hochleitner BW, Kafka R, Spechtenhauser B, et al. Renal allograft rupture is associated with rejection or acute tubular necrosis,but not with renal vein thrombosis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc – Eur Ren Assoc. 2001;16(1):124-127. doi:10.1093/ndt/16.1.124
- Dahle DO, Skauby M, Langberg CW, Brabrand K, Wessel N, Midtvedt K. Renal Cell Carcinoma and Kidney Transplantation: A Narrative Review. Transplantation. 2022;106(1). https://journals.lww.com/transplantjournal/Fulltext/2022/01000/Renal_Cell_Carcinoma_and_Kidney_Transplantation__A.18.aspx
- Eccher A, Girolami I, Motter JD, et al. Donor-transmitted cancer in kidney transplant recipients: a systematic review. J Nephrol. 2020;33(6):1321-1332. doi:10.1007/s40620-020-00775-4
- Lubetzky M, Tantisattamo E, Molnar MZ, et al. The failing kidney allograft: A review and recommendations for the care andmanagement of a complex group of patients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2021;21(9):2937-2949. doi:10.1111/ajt.16717
- Pham P-T, Everly M, Faravardeh A, Pham P-C. Management of patients with a failed kidney transplant: Dialysis reinitiation,immunosuppression weaning, and transplantectomy. World J Nephrol. 2015;4(2):148-159. doi:10.5527/wjn.v4.i2.148
- Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol. 2010;21(2):374-380. doi:10.1681/ASN.2009050480
- Schaefer HM, Helderman JH. Allograft Nephrectomy after Transplant Failure: Should It Be Performed in All Patients Returning to Dialysis? J Am Soc Nephrol. 2010;21(2):207-208. doi:10.1681/ASN.2009121262
- Kosmoliaptsis V, Gjorgjimajkoska O, Sharples LD, et al. Impact of donor mismatches at individual HLA-A, -B, -C, -DR, and -DQ loci on the development of HLA-specific antibodies in patients listed for repeat renal transplantation. Kidney Int. 2014;86(5):1039-1048.
- Knechtle SJ. Kidney Transplantation – Principles and Practice E-Book / Stuart J. Knechtle, Lorna P. Marson, Peter J Morris. 8th.; 2019.
- Sutherland DE, Simmons RL, Howard RJ, Najarian JS. Intracapsular technique of transplant nephrectomy. Surg Gynecol Obstet. 1978;146(6):950-952.
- Touma NJ, Sener A, Caumartin Y, Warren J, Nguan CY, Luke PPW. Extracapsular versus intracapsular allograft nephrectomy: impact onallosensitization and surgical outcomes. Can Urol Assoc J = J l’Association des Urol du Canada. 2011;5(1):49-52. doi:10.5489/cuaj.10016
- Takase HM, Contti MM, Nga HS, et al. Nephrectomy Versus Embolization of Non-Functioning Renal Graft: A SystematicReview with a Proportional Meta-Analysis. Ann Transplant. 2018;23:207-217. doi:10.12659/AOT.907700
- Yeh L-M, Chiu SY-H, Lai P-C. The Impact of Vascular Access Types on Hemodialysis Patient Long-term Survival. Sci Rep. 2019;9(1):10708. doi:10.1038/s41598-019-47065-z
- Almasri J, Alsawas M, Mainou M, et al. Outcomes of vascular access for hemodialysis: A~systematic review and meta-analysis. J Vasc Surg. 2016;64(1):236-243.
- Chan MR, Oza-Gajera B, Chapla K, et al. Initial vascular access type in patients with a failed renal transplant. Clin J Am Soc Nephrol. 2014;9(7):1225-1231. doi:10.2215/CJN.12461213
- Evans RDR, Bekele S, Campbell SM, et al. Assessment of a Dedicated Transplant Low Clearance Clinic and Patient Outcomes on Dialysis After Renal Allograft Loss at 2 UK Transplant Centers. Transplant Direct. 2018;4(6). https://journals.lww.com/transplantationdirect/Fulltext/2018/06000/Assessment_of_a_Dedicated_Transplant_Low_Clearance.4.aspx
- Perl J, Hasan O, Bargman JM, et al. Impact of Dialysis Modality on Survival after Kidney Transplant Failure. Clin J Am Soc Nephrol. 2011;6(3):582-590. doi:10.2215/CJN.06640810
Appendix 4.2 Distress Thermometer
Appendix 4.3 Work & Social Adjustment Scale
Appendix 4.4 Medication Adherence Report Scale 5
5. PAEDIATRICS
Statements of Recommendation
We recommend that:
- Paediatric patients with a failing allograft (eGFR<25ml/min/1.73m2) are managed by multidisciplinary teams involving low clearance and transplant nephrologists, dietitians, psychologists, social workers, clinical nurse specialists, transplant surgeons and additional local services as required. (1C)
- Where the allograft is failing, there is maintenance of immunosuppression (IS) to preserve residual kidney function and minimise sensitisation. (1C)
- Allograft nephrectomy is not routinely performed and if needed, it is discussed within the multidisciplinary team and the decision is made individually for each patient based on clinical circumstances. (1C)
- The target for systolic blood pressure is below the 90th centile for height, age and gender (with a long-term goal to achieve control near the 50th percentile for age). (1C)
- Patients with established hypertension have annual surveillance with an echocardiogram. (1C)
- Haemoglobin levels are maintained within the normal range (recommended range 100 – 130 g/L). (1C)
- Correction of acidosis, maintenance of phosphate and PTH levels close to the normal range. (1C)
- Children in a high-risk category for development of diabetes mellitus should have personalised surveillance, which would usually include 3 monthly measurements of HbA1c level. (1D)
- Children considered at low risk for the development of diabetes mellitus should have their HbA1C measured on an annual basis. (ungraded)
- All patients have regular monitoring of HLA antibodies. Frequency of testing will be led clinically and by the local team. (ungraded)
- Growth and nutrition surveillance to be evaluated at each clinic visit. (1B)
- Completion of all vaccines according to national guidelines (except live vaccines if still taking or within three months of cessation of IS). (1B)
- If indicated, recipients receive an annual review by the bladder/urology teams with regular bladder function assessments. (1D)
We suggest that:
- The back of the hand should be used for phlebotomy where possible to allow preservation of limb and central vasculature for future use. (1C)
- Early consideration for arterio-venous fistula for those patients approaching dialysis. (1D)
- Proteinuria is monitored three monthly. (1C)
- An individualised approach to work up for the next transplant in each patient based on HLA sensitisation, origin of next kidney (deceased or living), cause of previous allograft loss and underlying aetiology of end-stage kidney disease. (ungraded)
Suggestions for Future Research:
- What is the effect on sensitisation of complete IS withdrawal +/- nephrectomy compared to continuation of maintenance IS +/- nephrectomy?
- What is the effect on sensitisation of continuing calcineurin inhibitor (CNI) + anti-metabolite compared to CNI monotherapy?
Rationale
5.1 Introduction
There are no current guidelines or recommendations for the management of paediatric recipients with a failing kidney transplant. Compared to adult recipients, the management of children and adolescents with a kidney transplant involves additional considerations, and these can become more apparent should the transplant function decline. A child or adolescent with a failing transplant faces unique developmental and psychosocial challenges in addition to their medical management.1
The leading causes of end stage kidney disease (ESKD) in childhood differ substantially from those in adults and may also be relevant when considering the cause of transplant failure. The most common causes of ESKD (approximately 40%) are Congenital Anomalies of the Kidney and Urinary Tract (CAKUT, including posterior urethral valves and renal dysplasia with or without vesico-ureteric reflux, VUR).2 Hereditary kidney diseases are also a significant cause, including polycystic kidney disease, nephronophthisis, cystinosis, congenital nephrotic syndrome, primary hyperoxaluria, atypical haemolytic uraemic syndrome (aHUS), some glomerulopathies and familial focal segmental glomerulosclerosis (FSGS).
5.1.1. Defining a failing graft
There is no unifying definition of when the allograft is failing. Some propose that this is defined at the point that the patient restarts dialysis. Most transplant professionals would consider an allograft to be failing once the estimated GFR is less than 20 ml/minute/1.73m2 with some making the argument for it being below 25 ml/minute/1.73m2. Regardless of which definition is used, children and adolescents with a failing kidney transplant need special considerations and a multidisciplinary approach.
For children in particular, a pre-emptive second kidney transplant should be considered the gold standard of care. This approach would reduce complications associated with dialysis and would allow children to maintain their education and social interactions which are of utmost importance in adolescence and young adulthood.3
5.1.2. Causes of graft failure in children
In the UK, outcomes for paediatric kidney transplant recipients (KTRs) have improved significantly over recent years, mainly because of improvements in surgical techniques leading to a reduction in early allograft loss. The youngest recipients are at the highest risk for graft failure in the early post-operative period. The reasons for this include vascular complications and thrombosis particularly in small recipients (weight less than 15kg at the time of transplant) due to the donor-recipient size mismatch or in recipients with pre-existing vascular abnormalities (absence of the main vessels including aorta or inferior vena cava). Additionally, advances in IS regimens, improvements in HLA matching, treatment of recurrent disease and reduction in cold ischaemia time have all contributed to the improvements in allograft survival. However, as in adults, the most commonly reported cause of graft loss after the first year among paediatric KTRs is chronic allograft injury.4
Recurrence of primary diseases remains a risk for patients with non-genetic focal segmental glomerulosclerosis, mesangiocapillary glomerulonephritis (MCGN), aHUS, primary hyperoxaluria and rarely with membranous nephropathy.5 In comparison with patients with CAKUT (risk 14.4%), the 5- year risk of graft loss was significantly higher in patients with FSGS (25.7%) and MCGN (32.4%).6,7
Medication non-adherence is a leading cause of allograft loss in adolescents. The poorest allograft survival outcomes are among adolescents who have received a kidney transplant after long-term dialysis.8,9
Despite significant advances in infection monitoring and management, various infections (recurrent urinary tract infections, EBV, BKVAN) remain important risk factors for allograft failure. Post-transplant lymphoproliferative disease and malignancy may also lead to graft loss.
5.1.3. Epidemiology
All current renal registries for children only report outcomes for children with a functioning transplant and those on dialysis. There are no reports on the incidence and prevalence of paediatric KTRs with failing allografts, most likely due to the lack of a universal definition of a failing transplant in childhood.
5.2 Overall aims for the management of CKD (T)
The overall aims of the management of children with CKD (T) are to:
- Slow progression of kidney dysfunction.
- Maintain fluid and electrolyte balance.
- Allow adequate physical growth and psychosocial development.
- Maintain mineral bone homeostasis.
It is also imperative to preserve limb and central vasculature as much as possible, to ensure adequate options for haemodialysis if required.
Special considerations which should be tailored to each patient’s individual needs are discussed below.
5.2.1 Hypertension and cardiovascular disease
Hypertension after kidney transplantation is a frequent occurrence in paediatric KTRs. The aetiology is multifactorial, and it is an established independent risk factor for a decline in allograft function and loss. As hypertension contributes to a significant burden of cardiovascular disease (CVD)-related morbidity and mortality in this population, it is essential that it is diagnosed early and treated appropriately.10 With progressive allograft dysfunction, the risk for salt and water retention and hypertension increases.
We recommend:
Blood pressure surveillance:11,12
- Evaluation for symptomatic consequences of high blood pressure including headaches and visual symptoms.
- Blood pressure measurement at each clinical encounter.
- Use of centile charts based on American Academy of Paediatrics Hypertension Guideline from 2017.
- 24-hour ambulatory blood pressure monitoring (ABPM) to identify and monitor hypertension with ongoing annual surveillance. Patients younger than 5 years of age might need day admission to have serial BP checked as are unlikely to tolerate 24-hour ABPM.
- Patients with established hypertension should also have annual surveillance with an echocardiogram.
Blood pressure management:13
- The initial aim is for systolic blood pressure < 90th centile for height, age and gender (with a long-term goal to achieve control near the 50th percentile for age).
- Patient and parent or carer education on low-salt diet and regular exercise with maintenance of a healthy weight. Lowering the salt content in diet has been shown to improve systemic hypertension in the paediatric population.
- Investigating potentially correctable causes of hypertension, such as transplant renal artery stenosis (TRAS). If present and there is remaining allograft function or severe hypertension (not controlled on two medications), endovascular or surgical interventions should be considered in discussion with interventional radiology and transplant surgical teams.
- There is no clear choice of preferred antihypertensive medications for children with failing kidney transplants and hypertension. However, recurrence of disease, accompanying proteinuria, water and salt overload should be taken into consideration when choosing medication. Careful monitoring is recommended if blockade of the renin-angiotensin system is initiated.
5.2.2 Anaemia
Anaemia is present in a significant proportion of paediatric KTRs. Haemoglobin levels are strongly associated with allograft dysfunction, being the lowest in paediatric kidney transplant recipients with CKD stage V.14 Anaemia contributes to ongoing hypoxic injury and inflammation in the allograft, itself contributing to a further deterioration in allograft function.15
We recommend:
Anaemia surveillance:
- Evaluation for symptoms of anaemia in every outpatient clinic visit including tiredness, shortness of breath and lethargy.
- Blood tests: Full blood count (including reticulocyte count), every clinic; iron studies, three monthly.
Anaemia management:
- Aim to maintain haemoglobin levels within the normal range (dependent on local ranges) (usual range 100-130 g/L).
- Commence iron supplementation early if evidence of iron depletion.
- Commence Erythropoietin Stimulating Agents (ESA) when sustained reduction in reticulocyte count seen on surveillance.
- Consider reduction of anti-metabolites (azathioprine/MMF), especially in children with CKD (5T). Ongoing risks of sensitization need to be considered.
5.2.3 Optimisation of growth
Children and young adults with failing allografts need special attention paid to their growth and bone health as physical growth is highest in childhood. Ongoing inflammation from a failing allograft can have a detrimental effect on growth with ongoing protein energy malnutrition adding to challenges. Hyperparathyroidism is an independent risk factor for allograft dysfunction after kidney transplantation. Hence, it is vital to optimize bone and growth in the failing graft cohort.16,17
We recommend:
Growth and Nutrition Surveillance:
- Auxology at every outpatient visit (including height and weight).
- Review of symptoms of poor nutrition/worsening electrolytes (morning nausea or vomiting).
- Blood tests: Renal function, bone profile (calcium, phosphate, albumin, alkaline phosphatase), liver function, bicarbonate, every clinic; PTH, three monthly; vitamin D, annually.
Growth and Nutrition Management:
- The overall aim of management is to achieve optimal growth rate and maximise growth potential.
- Review of blood tests and correction of acidosis, maintenance of phosphate and PTH levels as close to the normal range as possible. Correction of anaemia as above.
- Involvement of renal dietitian.
5.2.4 Risk of diabetes
The risk of hyperglycaemia and diabetes is increased in paediatric KTRs mainly due to maintenance IS (steroids and tacrolimus) and in certain underlying conditions (e.g HNF1β mutations). Children from Black and Asian heritage are at an increased risk of developing hyperglycaemia or diabetes requiring treatment. As poorly controlled diabetes accelerates deterioration of allograft function, specialised care is required for prevention and timely management.18
We recommend:
Diabetes Surveillance:
- Children in a high-risk category as above, should have personalised surveillance, which would usually include 3 monthly measurements of HbA1c levels.
- Children considered at low risk for the development of diabetes mellitus should have their HbA1C measured on an annual basis.
- Ongoing dietetic support.
Diabetes management:
- Treatment should be guided by endocrine and dietetic teams.
- Reduction of IS, especially steroids, should be considered early. Again, this should be personalised depending on allograft function, HLA sensitisation and individual risks to the patient.
5.3. Immunosuppression
Currently, there are no uniform recommendations on the management of IS in children with failing allografts. Recent guidelines by the UK paediatric transplant group on IS give clear guidance on approaches for the first kidney transplant but do not address the failing allograft cohort separately. When optimising IS for this cohort of paediatric patients, a balance needs to be found between ensuring that sensitisation remains low (as re-transplantation would be recommended for the majority of patients) whilst minimizing complications.19,20 Evidence advising modulation of IS regimens is discussed in more detail in section 2 of this guideline (above).
Most paediatric KTRs’ maintenance IS falls into one of three categories:
- CNI and Mycophenolate Mofetil (MMF).
- CNI, azathioprine +/- steroids.
- CNI and steroids.
Generally, unless contraindicated, for patients with declining kidney allograft function with eGFR <25ml/min/1.73m2and those on dialysis, CNIs should be continued aiming for levels 5-7 mg/L (tacrolimus). Withdrawal and cessation of steroids should be considered early to allow for better growth. Anti-metabolites should be continued on lower doses (for example 0.5-1mg/kg once daily azathioprine and 150mg/m2 twice daily MMF).
In some situations, complete withdrawal of IS will need to be considered (severe clinically symptomatic infections, PTLD, persistently high levels of EBV). As always, the patient’s overall health and holistic approach should guide management.
Immunosuppression for a second or subsequent kidney transplant:
Some paediatric KTRs with failed allografts will have higher levels of sensitisation and/or preformed antibodies which might complicate HLA matching for future transplants. Limited reports in the literature suggest good outcomes with the use of Alemtuzumab as an induction agent, and maintenance with a CNI, MMF and slow weaning of steroids. We recommend an individualized approach to each patient based on HLA sensitisation, origin of next kidney (deceased or living), cause of previous allograft loss and underlying aetiology of ESKD.21,22
Patients with a failing antibody incompatible transplant:
These patients are very complex and their management should be in discussion with centres with established expertise and experience in antibody incompatible transplantation.23 This might require collaboration with adult transplant centres for planning of future transplants.
5.4 Vaccination and infection
Infections are one of the leading causes of morbidity and mortality following solid organ transplantation and children are at particular risk due to the immaturity of their immune system. Consequently, there should be a focus on evaluating and completing vaccination according to local guidelines. Despite their recognized vulnerability, vaccination rates in kidney transplant recipients are recognized to be suboptimal.24
We recommend:
- Completion of all vaccines according to national guidelines (except live vaccines if still taking or within three months of cessation of IS).
- Additional vaccines for immunocompromised patients as per national guidelines (e.g. influenza, pneumococcus, COVID 19, HPV).
- If treatment with Eculizumab is anticipated post-transplant, the patient should receive meningococcal vaccination with both a tetravalent A, C, W and Y conjugated vaccine and the multi-component serogroup B vaccine.
5.5 Surgical aspects
5.5.1 Transplant Nephrectomy
There are no recommendations for the timing and indications for transplant nephrectomy. A recent review did not find any significant demographic differences between those who underwent transplant nephrectomy and those who did not. The commonest reasons for nephrectomy are allograft tenderness and recurrent infection. Patients that underwent nephrectomy were more likely to have a prior diagnosis of rejection within three months. Nephrectomy of allografts did not affect time to re-listing for a second kidney transplant or donor source at re-transplantation but significantly decreased time to and incidence of complete cessation of immunosuppression post-graft failure.24Following transplant nephrectomy of the first allograft, recipients were significantly more likely to have rejection after re-transplantation and multiple episodes of rejection in the first year after re-transplant.25
We recommend that indications for allograft nephrectomy are discussed within multidisciplinary teams and made individually for each patient based on clinical circumstances.26,27
5.5.2 Bladder management
Children with CAKUT can continue to have bladder issues post-transplant. Once immunosuppressed, they may have an increased tendency to develop urinary tract infections.
Bladder augmentation or another surgical intervention (for example ureteric re-implantation or ureterostomy) may be required post-transplantation.
It is important that these children continue to empty their bladders completely. If they have an augmented bladder or a Mitrofanoff, they need to continue to catheterise regularly and completely.28
Constipation also needs to be avoided as it affects bladder emptying. Failing to comply with bladder hygiene can lead to further recurrent UTIs and worsening of allograft function.
We recommend an annual review by the bladder/urology teams with regular bladder function assessments to understand bladder capacity as the children continue to grow and bladder dynamics change. Transplant and low-clearance teams should continue to educate patients and families in ways understandable to them.
5.6. Vascular access/ Dialysis options
Children with ESKD have a lifetime of moving between transplant and dialysis teams. It is therefore imperative that when their transplant fails, they have a choice of dialysis modality which best suits them and their lifestyle. Measures to preserve sites for vascular access, from the time of diagnosis, is the key to enabling care providers to offer this choice. The back of the hand should be used for phlebotomy where possible. This will allow preservation of limb and central vasculature for future use.
Continued patient and staff education is crucial for these vulnerable children.
For children who are deemed suitable candidates for fistula formation, early referral to the vascular access team should be considered. Children with failing transplants are recommended to have an assessment with the vascular access team to allow optimisation of their choices.
5.7. Transition to adult services
Numerous studies have illustrated the importance of effective transition in empowering young adults to manage their own health. This is particularly important in young adults with a failing transplant. The transition process should be individually tailored and should enable them to understand their ongoing management, the workings of the health system and how to navigate it.29
Consideration of any learning difficulties and disabilities is also important when transitioning care.
For the subgroup of recipients with urological issues, it is essential that an appropriate adult urologist is concurrently identified to oversee their transition. This is to ensure ongoing regular check-ups and maintenance input such as catheter management is made for patients with abnormal bladders. As the patient matures, body habitus changes, hence revision of procedures such as a Mitrofanoff may be required.30
Potential adherence, social and psychological issues need to be explored in detail from the point of identification of the failing transplant and appropriate support needs to be put in place for the pre-, -peri- and post-transition period. Readiness for re-transplantation also needs to be addressed often requiring multidisciplinary input involving Young Adult Workers (YAW) and Psycho-social teams.
5.8. Multi-disciplinary approach
It is imperative that children with failing allografts receive ongoing support from not only the clinical and dietetic teams but also from psychosocial teams. A failing allograft adds a huge psychological burden not only on the young person but also the wider family. No quality-of-life data are currently available for children with failing allografts which must be a priority for future studies.31
References
- Lofaro D. et al. Multicenter Study. Identification of subgroups by risk of graft failure after paediatric renal transplantation: application of survival tree models on the ESPN/ERA-EDTA Registry Nephrol Dial Transplant. 2016 Feb;31(2):317-24.
- Harada R. et al. Epidemiology of pediatric chronic kidney disease/kidney failure: learning from registries and cohort studies. Pediatr Nephrol. 2022 Jun;37(6):1215-1229.
- Phonphok K. et al. Time to second kidney transplantation in young adults after failed pediatric kidney transplant. Pediatr Transplan. 2020 Nov;24(7):e13800.
- Ashore IF. et al. Non-immunologic allograft loss in pediatric kidney transplant recipients. Review Pediatr Nephrol. 2019 Feb;34(2):211-222.
- Van Stralen KJ. et al. Impact of graft loss among kidney diseases with a high risk of post-transplant recurrence in the paediatric population. Nephrol Dial Transplant. 2013 Apr;28(4):1031-8.
- Graves RC. et al. Kidney retransplantation in children following rejection and recurrent disease. Pediatr Nephrol. 2016 Dec;31(12):2235-2247.
- Cachat P. et al. Disease recurrence in paediatric renal transplantation. Review Pediatr Nephrol. 2009 Nov;24(11):2097-108.
- Ellis E. Et al. Comparative Study. Factors related to long-term renal transplant function in children. Pediatr Nephrol. 2008 Jul;23(7):1149-55
- Lofaro D. et al. Identification of subgroups by risk if graft failure after paediatric renal transplantation: application of survival tree models on the ESPN/ERA-EDTA Registry. Nephrol Dial Transplant. 2016;31(2):317-24.
- Mitsnefes MM. et al. Hypertension and end-organ damage in pediatric renal transplantation. Pediatr Transept. 2004;8(4):394-9.
- Wuhl E. et al. German Working Group on Pediatric Hypertension. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens (2002) 20(10):1995–2007.
- Krmar RT. et al. Clinical value of ambulatory blood pressure in pediatric patients after renal transplantation. Pediatr Nephrol. 2018 Aug;33(8):1327-1336.
- Mitsnefes MM. Et al. Short-term pediatric renal transplant survival: blood pressure and allograft function. Pediatr Transplant 2001: 5: 160–165.
- Krischock LA. Et al. Anemia in children following renal transplantation-results from the ESPN/ERA-EDTA Registry. ESPN/ERA-EDTA Registry. Pediatr Nephrol. 2016;31(2):325.
- Lubetzky M. Et al. The Failing Kidney Allograft: a Review and recommendations for the care and management of complex group of patients. Am J Transplant. 2021 Sept;21(9):2937-2949.
- Franke D. Et al. Kidney transplantation fails to provide adequate growth in children with chronic kidney disease born small for gestational age. Pediatr Nephrol. 2017 Mar;32(3):511-519.
- Franke et al. Patterns of growth after kidney transplantation among children with ESRD. Clin J Am Soc Nephrol. 2015 Jan 7;10(1):127-34.
- Mehls O. Et al. Growth hormone treatment after renal transplantation: a promising but underused chance to improve growth. Editorial Pediatr Nephrol. 2013 Jan;28(1):1-4.
- Lubetzky M. et al. The failing kidney allograft: A review and recommendations for the care and management of a complex group of patients. Pediatr Transplant. 2004 Dec;8(6):561-4.
- McCaffrey et al. CMV, EBV, JCV and BKV infection and outcome following kidney transplantation in children initiated on a corticosteroid-minimisation immunosuppressive regimen. Pediatr Nephrol. 2021;36(10);3229-40.
- Kim I. Et al. Risk factors for the development of antibody-mediated rejection in highly sensitized pediatric kidney transplant recipients. Pediatr Transplant. 2017 Dec;21(8).
- Foster BJ. Et al. Impact of HLA mismatch at first kidney transplant on lifetime with graft function in young recipients. Am J Transplant. 2014 Apr;14(4):876-85.
- Dudley et al. Clinical practice guidelines standardisation of immunosuppressive and anti-infective drugs regimens in UK paediatric renal transplantation: the harmonisation programme. BMC Nephrology Sept 2021.
- Fox et al. Vaccinations in pediatric kidney transplant recipients. Pediatr Nephrol. 2019;34(4):579-91.
- Minson S. Et al. Nephrectomy for the failed renal allograft in children: predictors and outcomes. Pediatr Nephrol. 2013 Aug;28(8):1299-305.
- Verghese P. Et al. Practice patterns and influence of allograft nephrectomy in pediatric kidney re-transplantation: A pediatric nephrology research consortium study. Pediatr Transplant. 2021 Sep;25(6):e13974.
- Phillips B. Et al. Graft nephrectomy in children. Pediatr Nephrol. 2018 Jun;33(6):947-955.
- Verghese et al. Practice patterns and influence of allograft nephrectomy in pediatric kidney re-transplantation: A pediatric nephrology research consortium study. Pediatr Transplant. 2021;25(6):e13974.
- Gupta M. Et al. Repeat Kidney Transplantation After Failed First Transplant in Childhood: Past Performance Informs Future Performance. Transplantation. 2015 Aug;99(8):1700-8.
- Foster B. Et al. Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol. 2015 Apr;30(4):567-76.
- Kreuzer M. Et al. Survey on Management of Transition and Transfer From Pediatric- to Adult-based Care in Pediatric Kidney Transplant Recipients in Europe. Transplant Direct. 2018 Jun 1;4(7):e36
6. LIFESTYLE AND THE FAILING KIDNEY TRANSPLANT
Statements of recommendation
We recommend that:
- Nutritional management of the complications of CKD aligns with the recommendations of the UKKA guideline. (1C)
- Recipients with an eGFR <20ml/min/1.73m2 are referred to a renal dietitian. (1D)
- A healthy weight is maintained to minimise cardio-metabolic risk factors. (1C)
- Where appropriate, achievable weight loss post is supported, using sustainable, personalised lifestyle changes, to reduce cardio-metabolic risk factors. (1B)
- Salt intake is limited to 6g per day to help reduce blood pressure. (1A)
- Lifestyle interventions are embedded with behaviour change technique(s) or behavioural therapies such as Cognitive Behavioural Therapy which have an underlying evidence-based theory for recipients with a failing kidney transplant. These must be reinforced continuously or periodically. (1B)
We suggest that:
- Dietary patterns that reflect principles of plant-based diets to reduce cardio-metabolic risk factors. (1C)
Research recommendations:
No specific targeted studies have been conducted in the failing kidney transplant setting. Further research is warranted regarding lifestyle interventions for failing kidney transplant recipients.
Questions for evidence review:
- What degree of weight loss (if any) is recommended in adults who are overweight or obese, with a failing kidney transplant in relation to reducing cardio-metabolic risk factors and relisting for transplantation?
- What dietary patterns are associated with cardiovascular risk improvement (e.g. Dietary Approaches to Stop Hypertension (DASH); Mediterranean eating patterns) in adults with a failing kidney transplant in relation to reducing cardio-metabolic risk factors?
- What behaviour change techniques/therapies can be used by healthcare professionals and kidney transplant recipients to support making meaningful changes to lifestyle for improved health outcomes?
Rationale
6.1 Introduction
“A healthy lifestyle is a way of living that lowers the risk of being seriously ill or dying early”. 1 The term “lifestyle” usually encompasses the following aspects (amongst others): diet, exercise, smoking and alcohol. Lifestyle is the result of choices that individuals make, for example, their food choices. Choices are in turn influenced by an individual’s cultural, socioeconomic and political environment. 2 Therefore, lifestyle influences post-transplant health and underpins optimisation of post-transplant health.
Benefits of a healthy lifestyle:
- Minimise post-transplantation cardio-metabolic risks of:
- Unintentional weight gain
- Post-Transplantation Diabetes Mellitus
- Hypertension
- Dyslipidaemia
- Major adverse cardiovascular events
- Improve wellbeing (quality of life and mental health)
- Improve the opportunity for re-transplantation
In this guideline, we consider the randomised controlled trial lifestyle interventions as well as observational cohort study evidence in adult kidney +/- pancreas transplant recipients that can lead to improvement in cardiometabolic risk factors after transplantation. Lifestyle interventions involving participants with a failing kidney transplant are non-existent, so these guidelines draw on the available evidence in renal transplantation more widely. However, declining eGFR is independently associated with cardiovascular disease risk and therefore lifestyle advice is pertinent to this population.3 Lifestyle changes regarding weight loss and cardio-metabolic disease modifications can be relevant to maintaining suitability for re-listing for a transplant.
The factors that will be explored in further detail in these guidelines are dietary patterns, weight management and their impact on wellbeing and cardio-metabolic health. Exercise, smoking and alcohol consumption have been covered in detail in the “Exercise and lifestyle in chronic kidney disease” clinical practice guideline published by the UK Kidney Association 4 and therefore will not be covered in this guideline. Additional guidance on nutritional assessment for patients with advanced CKD is available in the UKKA clinical practice guideline “Undernutrition in Chronic Kidney Disease”.4
6.2 Weight maintenance where appropriate, for recipients with a failing kidney
Cardiovascular disease is the primary cause of death after a kidney transplant 5 therefore minimising cardiovascular risk factors is important. Hypertension post kidney transplantation is common ranging from 50-80% 6. A higher weight post-transplant is associated with a higher prevalence of hypertension 7, as well as a greater incidence of post-transplant diabetes mellitus (PTDM).7,8 Therefore, weight maintenance post-transplant may result in significant benefits.
Weight gain after kidney transplantation is common and often associated with lipid abnormalities and increased fasting plasma glucose. The risk of obesity increases post kidney transplantation by 34% 9, which is associated with higher rates of PTDM, and contributes to higher mortality 10 as well as worse graft and patient survival.7
Most of post-transplant weight gain occurs in the first year post-transplant and affects a significant proportion of recipients. A Swiss cohort study found that 30% of recipients gained ≥5% of their 6 month post-transplant weight at 3 years post-transplantation 11 whilst another Brazilian retrospective cohort study found over 70% of kidney transplant recipients gaining weight at 1 year, with over 70% of these gaining ≥5% and over 40% gaining ≥10% of their baseline transplant weight. 12
Results from studies investigating pre-transplant body mass index (BMI) and its effect on the kidney transplant recipient and long-term graft survival have shown conflicting findings. Jary et al found there to be no impact of obesity on longer term outcomes.13 However, in other studies, significant weight gain post kidney transplant (defined as more than 10% after the first year and more than 20% after 2 years) has been associated with poor transplant outcomes such as worse graft function and post-operative complications.14–18 Others have identified a clear negative association between obesity and a decline in estimated glomerular filtration rate (eGFR) and allograft survival.19–21
6.3 Weight loss where appropriate, using sustainable, personalised lifestyle changes
Prevention of weight gain post transplantation due to the associated clinical and health risks 22 is preferable to weight loss interventions post transplantation (i.e. prevention rather than cure). However, in situations where weight gain does occur, lifestyle interventions can be useful. A recent Cochrane review on weight loss interventions for people who are overweight or obese with chronic kidney disease (which included a small number of studies in people with a renal transplant) found that “lifestyle-based weight loss interventions may result in weight loss and may have positive effects on diastolic blood pressure and low-density lipoprotein (LDL) cholesterol”.23
The randomised studies described here span over one to two years with variable success in relation to weight loss, which often was a surrogate outcome for improvement in cardio-metabolic risk factors. Orazio et al demonstrated a trend towards weight loss in a randomised study with a lifestyle intervention (Mediterranean and calorie deficit diet and physical activity) delivered over two years to people living with a kidney transplant, by a dietitian. However, there was no impact of or change in physical activity, potentially due to the lack of skills of the dietitian in advising on integrating physical activity into lifestyle.24 Henggeller et al conducted a randomised study (n=37) comparing an intensive weight management programme versus standard care with outcomes assessed after 6 months and found weight increased in both groups and no between-group differences were observed in the primary or any secondary outcomes (although standard care had a relatively high intensity input).25 Tzvetanov’s small study was unique in using behavioural interventions (including cognitive behavioural techniques and motivational interviewing) alongside nutritional and physical activity support over 12 months in a small sample of people with obesity post transplantation.26 The most impressive result from this study was that the intervention group had 100% retention compared to only 25% in the control group which might be due to the personalisation and behavioural focus of the programme. However, the sample size was small.
A single centre randomised study comparing the effects of an active (lifestyle advice delivered by renal dietitians using behaviour change techniques) versus passive intervention (leaflet advice alone) with kidney transplant recipients without diabetes found no change in surrogate markers of glucose metabolism over 6 months (primary outcome), despite improvements in weight and fat mass and a trend towards fewer cases of post-transplant diabetes.27 Six months may be too short a timeframe to result in a significant change in the primary outcome. In a follow up study 28, 2.5 years after the study was completed, any metabolic improvements were lost with no weight difference observed between active or passive intervention groups. This data suggests any impact from behaviour change must be reinforced continuously or periodically rather than a one-off intervention.
In summary, while lifestyle modification advice to kidney transplant patients remains a core part of post-transplant management, the evidence base for the efficacy of different lifestyle interventions on weight loss and therefore cardio-metabolic benefit is poor. In addition, no specific targeted studies have been conducted in the failing kidney transplant setting. There are some indicators that may contribute to the effectiveness of interventions: these should include a behavioural change component as well as a defined style of or approach to communication of advice and sustainability of outcomes should extend beyond 6-12 months which is critical when living with a chronic condition.
6.4 Limitation of salt intake
The recommendations supporting limiting salt consumption for people with a kidney transplant to reduce blood pressure are based on two randomised studies 29 and cohort studies 30,31 focusing on kidney transplant recipients. A recent update of a Cochrane review of salt intake for people with chronic kidney disease 32 as well as national UK Kidney Association commentary 33 both recommend a salt intake of less than 6g per day.
It is worth noting that KDIGO in their clinical practice guideline for the management of blood pressure in chronic kidney disease recommends <5g salt per day.34 They reflect that most of the studies in people with CKD focus on limiting salt to <5g or <6g of salt per day and that the decision to recommend <5g was to achieve “concordance across guidelines”. This guideline, however, highlights changes in lifestyle that are going to be sustainable as well as beneficial for health.
6.5 Behaviour change techniques
Behaviour change techniques are used to support the sustainability and effectiveness of lifestyle interventions. There are several behaviour change techniques 35 however, there are only a few randomised controlled trials that have employed one or more such techniques with kidney transplant recipients 25,27,36–40, such as motivational interviewing, prompt self-monitoring of behaviour/ behavioural outcome, feedback on performance, plan social support/social change amongst others. These behaviour change techniques are likely to have contributed to improvements in outcomes that are pertinent to weight related outcomes 27,38 or physical activity/function outcomes.36,38,40 “Second generation” behavioural therapies that incorporate additional cognitive strategies such as Cognitive Behavioural Therapy can be helpful in making sustainable changes in lifestyle. A small scale pilot study found 100% adherence (compared to 25% in the control group) to a 12 month rehabilitation program that incorporated cognitive behavioural therapy in kidney transplant recipients with a BMI of over 30kg/m2. 26 As highlighted above, any impact from behaviour change must be reinforced continuously or periodically rather than a one-off intervention. This would be consistent with evidence relating to the sustainability of behaviour change intervention for weight management in the general population. 41
6.6 Role of plant-based diets
Plant-based diets are defined as “dietary patterns that have a greater emphasis on foods derived from plants (such as fruits, vegetables, wholegrains, pulses, nuts, seeds and oils)” by the British Nutrition Foundation.42 They can include small amounts of animal foods such as meat, fish, poultry, eggs and dairy products. Examples of dietary patterns that are plant based are: the Mediterranean diet; the DASH diet; vegetarianism and veganism.
Published research with kidney transplant recipients and plant-based diets are limited to observational studies. A cohort population of between 468 and 632 kidney transplant recipients (>1 year) with a median follow-up of between 4 and 5.4 years has revealed associations between a Mediterranean Diet and a lower risk of transplant outcomes of graft failure, kidney function decline, graft loss 43 and diabetes post transplantation.44 The same cohort found an association between a DASH Score and lower risk of renal function decline and all-cause mortality.30 Furthermore, those with the highest tertile of DASH scores had improved cardio metabolic features: lower blood pressure, lower fasting triglycerides, and higher high-density lipoprotein (HDL) cholesterol concentrations, when compared to those in the lowest tertile. The consumption of more vegetables (but not fruit) was found to also be associated with a lower risk of post transplant diabetes, mediated by factors of the metabolic syndrome such as HDL cholesterol, triglycerides and waist circumference.45
In summary, a plant-based diet may be useful in improving transplant outcomes by targeting cardio-metabolic risk factors.
References
- WHO. Healthy Living – What Is a Healthy Lifestyle.; 1998.
- Booth SL, Sallis JF, Ritenbaugh C, et al. Environmental and societal factors affect food choice and physical activity: Rationale, influences, and leverage points. Nutr Rev. 2001;59(3 II):S21-S36. doi:10.1111/j.1753-4887.2001.tb06983.x
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073-2081. doi:10.1016/S0140-6736(10)60674-5
- Renal Association Clinical Practice Guidelines. Exercise and Lifestyle in Chronic Kidney Disease; 2021 https://ukkidney.org/sites/renal.org/files/Exercise%20and%20Lifestyle%20in%20CKD%20clinical%20practice%20guideline33_v4_FINAL_0.pdf; Undernutrition in Chronic Kidney Disease, 2019 (https://ukkidney.org/sites/renal.org/files/FINAL-Nutrition-guideline-June-2019-RNG-endorsed.pdf.
- Ying T, Shi B, Kelly PJ, Pilmore H, Clayton PA, Chadban SJ. Death after kidney transplantation: An analysis by era and time post-transplant. J Am Soc Nephrol. 2020;31(12):2887-2899. doi:10.1681/ASN.2020050566
- Weir MR, Burgess ED, Cooper JE, et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015;26(6):1248-1260. doi:10.1681/ASN.2014080834
- Lafranca JA, IJermans JNM, Betjes MGH, Dor FJMF. Body mass index and outcome in renal transplant recipients: A systematic review and meta-analysis. BMC Med. 2015;13(1):1-18. doi:10.1186/s12916-015-0340-5
- Sharif A, Cohney S. Post-transplantation diabetes-state of the art. Lancet Diabetes Endocrinol. 2016;4(4):337-349. doi:10.1016/S2213-8587(15)00387-3
- Nöhre M, Schieffer E, Hanke A, et al. Obesity After Kidney Transplantation—Results of a KTx360°Substudy. Front Psychiatry. 2020;11:399. doi:10.3389/FPSYT.2020.00399/BIBTEX
- Ahmadi SF, Zahmatkesh G, Ahmadi E, et al. Association of Body Mass Index with Clinical Outcomes in Non-Dialysis-Dependent Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2016;6(1):37-49. doi:10.1159/000437277
- Beckmann S, Nikolic N, Denhaerynck K, et al. Evolution of body weight parameters up to 3 years after solid organ transplantation: The prospective Swiss Transplant Cohort Study. Clin Transplant. 2017;31(3):1-11. doi:10.1111/ctr.12896
- Forte CC, Pedrollo EF, Nicoletto BB, et al. Risk factors associated with weight gain after kidney transplantation: A cohort study. PLoS One. 2020;15(12 December):1-11. doi:10.1371/journal.pone.0243394
- Järv L, Pechter Ü, Kuudeberg A, Lember M, Ots-Rosenberg M. Effect of Pretransplant Body Mass Index on Kidney Transplant Recipient and Graft Long-term Survival. Transplant Proc. 2021;53(10):2879-2887. doi:10.1016/j.transproceed.2021.09.040
- Erturk T, Berber I, Cakir U. Effect of Obesity on Clinical Outcomes of Kidney Transplant Patients. Transplant Proc. 2019;51(4):1093-1095. doi:10.1016/j.transproceed.2019.02.012
- Camilleri B, Bridson JM, Sharma A, Halawa A. From chronic kidney disease to kidney transplantation: The impact of obesity and its treatment modalities. Transplant Rev. 2016;30(4):203-211. doi:10.1016/j.trre.2016.07.006
- Molnar MZ, Streja E, Kovesdy CP, et al. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant. 2011;11(4):725-736. doi:10.1111/j.1600-6143.2011.03468.x
- Moreira TR, Bassani T, De Souza G, Manfro RC, Gonçalves LFS. Obesity in kidney transplant recipients: Association with decline in glomerular filtration rate. Ren Fail. 2013;35(9):1199-1203. doi:10.3109/0886022X.2013.819735
- Olarte IG, Hawasli A. Kidney transplant complications and obesity. Am J Surg. 2009;197(3):424-426. doi:10.1016/j.amjsurg.2008.11.021
- Bellini MI, Koutroutsos K, Nananpragasam H, Deurloo E, Galliford J, Herbert PE. Obesity affects graft function but not graft loss in kidney transplant recipients. J Int Med Res. 2020;48(1):1-9. doi:10.1177/0300060519895139
- Zaydfudim V, Feurer ID, Moore DR, Moore DE, Pinson CW, Shaffer D. Pre-transplant Overweight and Obesity Do Not Affect Physical Quality of Life after Kidney Transplantation. J Am Coll Surg. 2010;210(3):336-344. doi:10.1016/j.jamcollsurg.2009.11.009
- Hap K, Madziarska K, Hap W, et al. Are Females More Prone Than Males to Become Obese After Kidney Transplantation? Ann Transplant. 2019;24:57-61. doi:10.12659/AOT.912096
- Altheaby A, Alajlan N, Shaheen MF, et al. Weight gain after renal transplant: Incidence, risk factors, and outcomes. PLoS One. 2022;17(6 June):1-10. doi:10.1371/journal.pone.0268044
- Conley MM, McFarlane CM, Johnson DW, Kelly JT, Campbell KL, MacLaughlin HL. Interventions for weight loss in people with chronic kidney disease who are overweight or obese. Cochrane Database Syst Rev. 2021;2021(3). doi:10.1002/14651858.CD013119.pub2
- Orazio LK, Isbel NM, Armstrong KA, et al. Evaluation of Dietetic Advice for Modification of Cardiovascular Disease Risk Factors in Renal Transplant Recipients. J Ren Nutr. 2011;21(6):462-471. doi:10.1053/J.JRN.2010.12.002
- Henggeler CK, Plank LD, Ryan KJ, et al. A Randomized Controlled Trial of an Intensive Nutrition Intervention Versus Standard Nutrition Care to Avoid Excess Weight Gain After Kidney Transplantation: The INTENT Trial. J Ren Nutr. 2018;28(5):340-351. doi:10.1053/j.jrn.2018.03.001
- Tzvetanov I, West-Thielke P, D’Amico G, et al. A novel and personalized rehabilitation program for obese kidney transplant recipients. Transplant Proc. 2014;46(10):3431-3437. doi:10.1016/j.transproceed.2014.05.085
- Kuningas K, Driscoll J, Mair R, et al. Comparing glycaemic benefits of active versus passive lifestyle intervention in kidney allograft recipients: A randomized controlled trial. Transplantation. 2020;104(7):1491-1499. doi:10.1097/TP.0000000000002969
- Kuningas, K.; Driscoll J.;Mair, R.;Day E.;Sharif A. Short-term Healthy Lifestyle Intervention and Long-term Behavior Change After Kidney Transplantation: Findings From the CAVIAR Study. Am J Kidney Dis. 2022;Pre-proof.
- De Vries L V., Dobrowolski LC, Van Den Bosch JJON, et al. Effects of dietary sodium restriction in kidney transplant recipients treated with renin-angiotensin-aldosterone system blockade: A randomized clinical trial. Am J Kidney Dis. 2016;67(6):936-944. doi:10.1053/j.ajkd.2015.11.026
- Osté MCJ, Gomes-Neto AW, Corpeleijn E, et al. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am J Transplant. 2018;18(10):2523-2533. doi:10.1111/ajt.14707
- Van Den Berg E, Geleijnse JM, Brink EJ, et al. Sodium intake and blood pressure in renal transplant recipients. Nephrol Dial Transplant. 2012;27(8):3352-3359. doi:10.1093/ndt/gfs069
- McMahon EJ, Campbell KL, Bauer JD, Mudge DW, Kelly JT. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev. 2021;2021(6). doi:10.1002/14651858.CD010070.pub3
- Fish R, Chitalia N, Doulton T, et al. Commentary on NICE Guideline (NG136) “Hypertension in Adults: Diagnosis and Management” Including Proposals for Blood Pressure Management in Patients with Chronic Kidney Disease.; 2021.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3S):S1-S87.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol Heal. 2011;26(11):1479-1498. doi:10.1080/08870446.2010.540664
- Serper M, Barankay I, Chadha S, et al. A randomized, controlled, behavioral intervention to promote walking after abdominal organ transplantation: results from the LIFT study. Transpl Int. 2020;33(6):632-643. doi:10.1111/tri.13570
- Schmid-Mohler G, Zala P, Graf N, et al. Comparison of a Behavioral Versus an Educational Weight Management Intervention after Renal Transplantation: A Randomized Controlled Trial. Transplant Direct. 2019;5(12):1-9. doi:10.1097/TXD.0000000000000936
- Castle EM, Dijk G, Asgari E, et al. The Feasibility and User-Experience of a Digital Health Intervention Designed to Prevent Weight Gain in New Kidney Transplant Recipients—The ExeRTiOn2 Trial. Front Nutr. 2022;9(May):1-18. doi:10.3389/fnut.2022.887580
- Hsiao CY, Lin LW, Su YW, Yeh SH, Lee LN, Tsai FM. The Effects of an Empowerment Intervention on Renal Transplant Recipients: A Randomized Controlled Trial. J Nurs Res. 2016;24(3):201-210. doi:10.1097/jnr.0000000000000115
- O’Brien T, Russell CL, Tan A, et al. A Pilot Randomized Controlled Trial Using SystemCHANGETM Approach to Increase Physical Activity in Older Kidney Transplant Recipients. Prog Transplant. 2020;30(4):306-314. doi:10.1177/1526924820958148
- Teixeira PJ, Marques MM. Health Behavior Change for Obesity Management. Obes Facts. 2018;10(6):666-673. doi:10.1159/000484933
- British Nutrition Foundation. https://www.nutrition.org.uk/putting-it-into-practice/plant-based-diets/plant-based-diets/. Accessed November 1, 2022.
- Gomes-Neto AW, Osté MCJ, Sotomayor CG, et al. Mediterranean style diet and kidney function loss in kidney transplant recipients. Clin J Am Soc Nephrol. 2020;15(2):238-246. doi:10.2215/CJN.06710619
- Osté MCJ, Corpeleijn E, Navis GJ, et al. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res Care. 2017;5(1):1-8. doi:10.1136/bmjdrc-2016-000283
- Gomes-Neto AW, Osté MCJ, Sotomayor CG, et al. Fruit and vegetable intake and risk of post trans plantation diabetes in renal transplant recipients. Diabetes Care. 2019;42(9):1645-1652. doi:10.2337/dc19-0224
7. CARDIOVASCULAR AND METABOLIC RISK MANAGEMENT
Statements of Recommendation
We recommend that:
- Aim for blood pressure target of <130/80 for kidney transplant recipients with individualized decision-making as clinically appropriate. (1B)
We suggest that:
- CCB or ACEi/ARB should be considered first-line treatment options for hypertension post kidney transplantation depending on the needs of the individual patient. (1A)
- Blood pressure treatment should be tailored to the individual patient. (ungraded)
Recommendations for future research:
- Investigate the effects of different antihypertensive agents on graft survival and cardiovascular outcome in kidney transplant recipients with a failing allograft.
Recommendations for audit:
- Determination of the number of patients who have blood pressure control as suggested by the guideline.
- Identification of barriers to achieving these targets, for example, potential contributory factors to non-adherence (side effects, lack of understanding of benefits, pill burden).
Rationale
7.1 Introduction
There is a strong correlation between blood pressure control and graft outcome after kidney transplantation 1-4 with some (but not consistent) evidence that intervention to control blood pressure may improve outcomes.3-6 However, there are no prospective randomized controlled trials demonstrating the proven benefit of intervention or guiding target blood pressures.
7.2 Diagnosis of Hypertension Post-Transplantation
Hypertension in the renal transplant recipient is driven by both traditional and non-traditional risk factors. Vetromile et al 7 concluded on multivariate analysis of 811 recipients, that recipient age (p < 0.001), measured GFR (p = 0.037), albuminuria (p < 0.001), and metabolic syndrome (p = 0.007) were significantly associated with resistant hypertension. Weight gain is common post-transplantation which will exacerbate many of the elements of the metabolic syndrome.8 Aggressively controlling conventional cardiovascular risk factors in the treatment of hypertension through lifestyle changes is universally recommended and is considered in detail in section 6.
If a renal transplant recipient with previously well-controlled blood pressure develops new or worsening hypertension, consideration should be given to undertaking a secondary screen to look for physiological changes which may have triggered this. Sleep-disordered breathing has been noted to be a causative factor 9, especially in overweight patients. Tacrolimus has less impact on blood pressure than cyclosporine 10-13 and was established as the standard first-line treatment in the SYMPHONY trial. 14,15 Changing from a CNI to an mTOR inhibitor purely on the basis of cardiovascular risk is not recommended based on the higher risk of acute rejection.14, 15 Long-term steroid treatment is associated with the development of hypertension in the general population 16 but at the levels generally used for maintenance in transplant recipients, seems to be less clearly linked.17-19 Trials of withdrawal of steroids have shown no benefit on blood pressure control.20
Transplant renal artery stenosis can occur at any stage post-transplant. Early onset is most likely to be related to technical complications from surgery whereas later onset is due to the progression of underlying atheroma.21 No advantage has been shown of stenting over angioplasty alone in terms of outcome, but stenting may reduce the incidence of re-stenosis.22 Denervation of the native kidneys has been undertaken but only in a very small series 23, 24 providing limited evidence of safety or efficacy.
7.3 Target Blood Pressure
Although target blood pressure for chronic kidney disease (CKD) has been examined in a number of studies and recommendations have been made in national and international guidelines, there are fewer studies looking at ideal blood pressure targets in the kidney transplant population. In a study of over 24000 kidney transplant recipients, Opelz et al 4 showed that blood pressure <140 systolic is associated with better graft and patient survival. Inspired by the SPRINT trial 25, a study by Pagonas et al 5 compared a systolic blood pressure target of <130 with <140 in 815 kidney transplant recipients. After a median follow-up of 83.5 months, patients in the lower blood pressure group had significantly reduced mortality with a similar safety profile.
Both ACC/AHA 26 and KIDIGO 27 guidelines recommend a blood pressure target of <130/80 for the transplant population using standardized office blood pressure measurements. NICE CKD guideline 28 does not specify a target for kidney transplant recipients but recommends blood pressure <140/90 for CKD patients with ACR <70 and <130/80 for CKD patients with ACR >70.
We recommend a blood pressure target of <130/80 for kidney transplant recipients with individualized decision-making as clinically appropriate.
7.4 Pharmacotherapy
At present, there is no evidence to suggest that blood pressure treatment should be any different in kidney transplant recipients with deteriorating graft function. There are no RCTs investigating the effect of different antihypertensive agents on graft survival and cardiovascular risk specifically undertaken in kidney transplant recipients with a failing allograft.
A meta-analysis performed in 2020 looked at the effectiveness of different antihypertensive agents in kidney transplant recipients; however, it did not address hypertension treatment specifically in patients with failing allografts. None of the antihypertensive agents evaluated in this meta-analysis demonstrated a significant reduction in fatal or non-fatal cardiovascular events in the kidney transplant population, after taking into account the reduction in blood pressure.29
The role of RAS inhibitor treatment in reducing proteinuria, cardiovascular events, and blood pressure control in renal transplant recipients 30, 31, has been investigated in several studies. Other studies have reviewed their effect on patient and graft survival.32, 33 ACEi use appears to be associated with a decreased risk of graft loss in renal transplant recipients. However, this comes at the expense of a lower eGFR compared with controls which can be detrimental in patients with a failing allograft.29, 34 ACEi and/or ARB treatment benefits including increased graft survival, improved blood pressure control and reduced proteinuria may be outweighed by competing harms such as the increased risk of hyperkalemia, reduction in eGFR and worsening anaemia in patients with a failing allograft.
In contrast, treatment with dihydropyridine calcium channel blockers (CCBs) results in short-term preservation or improvement in renal function, as assessed by two pooled analyses of 13 and 15 RCTs on eGFR and serum creatinine, respectively reported by Pisano et al.29 This effect could be explained by vasodilation of the afferent arteriole and an increase in intraglomerular pressure. Data from 16 RCTs involving 1327 kidney transplant recipients suggest that the administration of CCBs, as compared with placebo or routine therapy, also reduces the risk of graft loss as seen with ACEi.29 Figure 1 summarizes the effect of CCB, ACEi and ARB treatment on various outcomes.29
There is very limited data in the literature regarding other antihypertensives such as a-blockers, b-blockers and diuretics. There are reports that b -blockers might be beneficial to long-term survival through better blood pressure control and cardiovascular event prevention, with minimal effect on renal function or proteinuria.35 However, there is evidence of possible metabolic side effects such as effects on glucose and lipid metabolism.36-38
Figure 1. Summary of effects of different drug use on key outcomes. The last column shows the effect when studies using ACEi and ARB are pooled together29
7.5 Management of Hyperlipidaemia and Hyperglycaemia (Including Post-Transplant Diabetes) in Renal Transplant Recipients
There is minimal data at present specific to the kidney transplant recipient with hyperlipidemia, with the majority being derived from the Assessment of Lescol in Renal Transplantation (ALERT) study and its extension studies.39-42 A joint publication by the Association of British Clinical Diabetologists (ABCD) and the Renal Association (RA) for the management of hyperlipidemia in patients with CKD43 recommends the same principles are extrapolated to the renal transplant recipient, taking into account the relevant drug interactions and we would concur with this.
The management of hyperglycemia in patients with CKD has been addressed by a recent excellent joint guideline issued by the ABCD/RA44 with earlier recommendations specific to post-transplant diabetes.45 In the absence of any new evidence, it is suggested that the same advice is followed. Below is a summary of the recommendations.
- We recommend treatment choices in the management of hyperlipidemia are consistent with those recommended by the ABCD/RA joint guidelines and BTS/Renal Association Clinical Practice Guideline Post-operative care in the Kidney Transplant Recipient namely:
- Statins should be continued or considered for all renal transplant recipients who have ESRD due to diabetic kidney disease (DKD) or who develop post-transplant diabetes mellitus (PTDM)
- Medication choices and doses should be individualized based on interactions with immunosuppressive medications; with atorvastatin 20mg daily recommended where indicated
- Aim to reduce total cholesterol to ≤4.0 mmol/L, non-HDL cholesterol to ≤2.5 mmol/L and LDL cholesterol to ≤2 mmol/L
- We recommend treatment choices in the management of hyperglycaemia are consistent with those recommended by the ABCD/RA joint guidelines namely:
- A formal diagnosis of PTDM can be made from six weeks post-transplantation using an oral glucose tolerance test (gold standard) or after twelve weeks using HbA1c >48mmol/L (>6.5%)
- The target HbA1c for people with diabetes and a renal transplant should be around 53 mmol/L (7%), individualized to the patient’s needs and preferences
- In patients with a stable eGFR >30 ml/min/1.73m2 and BMI >25 kg/m2, metformin should be considered first-line oral therapy for people with confirmed PTDM
- Individualization of immunosuppression based on the recipient’s immunologic and glycaemic risk must be taken as part of an overall strategy to improve long-term transplant outcome and any planned modification to attenuate this risk should be balanced against the risk for allograft rejection
References
- Mange KC, Cizman B, Joffe M, Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000; 283: 633-8
- Mange KC, Feldman HI, Joffe MM, Fa K, Bloom RD. Blood pressure and the survival of renal allografts from living donors. J Am Soc Nephrol. 2004; 15: 187-93
- Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney Int. 1998; 53 (1): 217-22
- Opelz G, Dohler B. Improved long-term outcomes after renal transplantation associated with blood pressure control. Collaborative Transplant Study. Am J Transplant. 2005; 5: 2725-31
- Pagonas N, Bauer F, Seibert FS, Seidel M, Schenker P, Kykalos S, Dürr M, Reinke P, Babel N, Viebahn R, Westhoff TH. Intensive blood pressure control is associated with improved patient and graft survival after renal transplantation. Sci Rep. 2019;9(1):10507
- Malhotra R, Katz R, Weiner DE, Levey AS, Cheung AK, Bostom AG. Blood Pressure, Chronic Kidney Disease Progression, and Kidney Allograft Failure in Kidney Transplant Recipients: A Secondary Analysis of the FAVORIT Trial. American Journal of Hypertension. 2019; 32 (9): 816-823
- Vetromile F, Pernin V, Szwarc I, Garrigue V, Delmas S, Mourad G, Fesler P. Prevalence and risk factors of noncontrolled and resistant arterial hypertension in renal transplant recipients. 2015; 99 (5): 1016-22
- Workeneh B, Moore LW, Nolte Fong JV, Shypailo R, Gaber AO, Mitch WE. Successful kidney transplantation is associated with weight gain from truncal obesity and insulin resistance. J Ren Nutr. 2019; 29: 548–55
- Mallamaci F, Tripepi R, D’Arrigo G, Panuccio V, Parlongo G, Caridi G, Versace MC, Parati G, Tripepi G, Zoccali C. Sleep-Disordered Breathing and 24-Hour Ambulatory Blood Pressure Monitoring in Renal Transplant Patients: Longitudinal Study. Journal of the American Heart Association. 2020; 9(13): e016237
- Margreiter R. Efficacy and safety of tacrolimus compared with ciclosporin micro emulsion in renal transplantation: a randomised multicentre study. Lancet. 2002; 359: 741–6
- Kramer BK, Del Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, Ortuno J, et al. Efficacy and safety of tacrolimus compared with ciclosporin A in renal transplantation: three-year observational results. Nephrol Dial Transplant. 2005; 23 (7): 2386–92
- Morales JM, Dominguez-Gil B. Impact of tacrolimus and mycophenolate mofetil combination on cardiovascular risk profile after kidney transplantation. J Am Soc Nephrol. 2006; 17: S296–303
- Seibert F, Behrendt C, Schmidt S, van der Giet M, Zidek W, Westhoff TH. Differential effects of cyclosporine and tacrolimus on arterial function. Transpl Int. 2011; 24: 708–15
- Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl J Med. 2007; 357: 2562–75
- Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U, Vanrenterghem Y, Daloze P, Halloran P. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009; 9(8): 1876-85
- Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017; 39: 2216–29
- Aziz F, Clark D, Garg N, Mandelbrot D, Djamali A. Hypertension guidelines: how do they apply to kidney transplant recipients. Transpl Rev. 2018; 32: 225–33
- Jackson SH, Beevers DG, Myers K. Does long-term low-dose corticosteroid therapy cause hypertension? Clin Sci. 1981; 61: 381s–3s
- Curtis JJ, Galla JH, Kotchen TA, Lucas B, McRoberts JW, Luke RG. Prevalence of hypertension in a renal transplant population on alternate-day steroid therapy. Clin Nephrol. 1976; 5(3): 123–7
- Pascual J, Royuela A, Galeano C, Crespo M, Zamora J. Very early steroid withdrawal or complete avoidance for kidney transplant recipients: a systematic review. Nephrol Dial Transplant. 2012; 27: 825–3
- Rajan DK, Stavropoulos SW, Shlansky-Goldberg RD. Management of transplant renal artery stenosis. Semin Interv Radiol. 2004; 21: 259–69
- Chen LX, De Mattos A, Bang H, Vu CT, Gandhi M, Alnimri M, et al. Angioplasty vs stent in the treatment of transplant renal artery stenosis. Clin Transpl. 2018; 32(4): e13217
- Dobrowolski LC, Bemelman FJ, Ten Berge IJ, van den Born BJ, Reekers JA, Krediet CT. Renal denervation of the native kidneys for drug-resistant hypertension after kidney transplantation. Clin Kidney J. 2015; 8: 79–81
- Schneider S, Promny D, Sinnecker D, Byrne RA, Muller A, Dommasch M, Wildenauer A, Schmidt G, Heemann U, Laugwitz KL, Baumann M. Impact of sympathetic renal denervation: a randomized study in patients after renal transplantation (ISAR-denerve). Nephrology Dialysis Transplantation. 2015; 30 (11): 1928-36
- Wright, J. T. Jr., Whelton, P. K. & Reboussin, D. M. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 374, 2294, https://doi.org/10.1056/NEJMc1602668 (2016)
- Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C. et al. ACC/AHA/AAPA/ABC/ ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology / American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Volume 99, issue 3S March 2021. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2021-BP-GL.pdf
- NICE guideline NG203 – chronic kidney disease: assessment and management (Aug 2021). Available at https://www.nice.org.uk/guidance/ng203 (accessed 18th Jan 2022)
- Pisano A, Bolignano D, Mallamaci Fet al. Comparative effectiveness of different antihypertensive agents in kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. 2020 May 1;35(5):878-887
- Paoletti E, Bellino D, Marsano L et al. Effects of ACE inhibitors on longterm outcome of renal transplant recipients. A randomized controlled trial. Transplantation 2013; 95: 889–895
- Mandelbrot DA, Alberu J, Barama A et al. Effect of ramipril on urinary protein excretion in maintenance renal transplant patients converted to sirolimus. Am J Transplant 2015; 15: 3174–3184
- Hiremath S, Fergusson DA, Fergusson N et al. Renin-angiotensin system blockade and long-term clinical outcomes in kidney transplant recipients: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2017; 69: 78–86
- Cross NB, Webster AC, Masson P et al. Antihypertensive treatment for kidney transplant recipients. Cochrane Database Syst Rev 2009; 3: CD00359
- Hiremath S, Fergusson D, Doucette S et al. Renin angiotensin system blockade in kidney transplantation: a systematic review of the evidence. Am J Transplant 2007; 7: 2350–2360
- Kuzmiuk-Glembin I, Adrych D, Tylicki L et al. Treatment of hypertension in renal transplant recipients in four independent cross-sectional analyses. Kidney Blood Press Res 2018; 43: 45–54
- AftabW, Varadarajan P, Rasool S et al. Beta and angiotensin blockades are associated with improved 10-year survival in renal transplant recipients. J Am Heart Assoc 2013; 2: e000091
- Suwelack B, Kobelt V, Erfmann M et al. Long-term follow-up of ACE inhibitor versus beta-blocker treatment and their effects on blood pressure and kidney function in renal transplant recipients. Transplant Int 2003; 16: 313–320
- Tylicki L, Biedunkiewicz B, Chamienia A et al. Randomized placebo-controlled study on the effects of losartan and carvedilol on albuminuria in renal transplant recipients. Transplantation 2006; 81: 52–56
- Holdaas, H.; Fellström, B.; Jardine, A. G.; Holme, I.; Nyberg, G.; Fauchald, P.; Grönhagen-Riska, C.; Madsen, S.; Neumayer, H.; Cole, E.; Maes, B.; Ambühl, P.; Olsson, A. G.; Hartmann, A.; Solbu, D. O.; Pedersen, T. R. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 2003, 361, 2024-2031.
- Holdaas, H.; Fellström, B.; Cole, E.; Nyberg, G.; Olsson, A. G.; Pedersen, T. R.; Madsen, S.; Grönhagen-Riska, C.; Neumayer, H. -.; Maes, B.; Ambühl, P.; Hartmann, A.; Staffler, B.; Jardine, A. G. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am. J. Transplant. 2005, 5, 2929-2936.
- Jardine, A. G.; Fellström, B.; Logan, J. O.; Cole, E.; Nyberg, G.; Grönhagen-Riska, C.; Madsen, S.; Neumayer, H.; Maes, B.; Ambühl, P.; Olsson, A. G.; Pedersen, T.; Holdaas, H. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am. J. Kidney Dis. 2005, 46, 529-536.
- Palmer, S. C.; Navaneethan, S. D.; Craig, J. C.; Perkovic, V.; Johnson, D. W.; Nigwekar, S. U.; Hegbrant, J.; Strippoli, G. F. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst Rev 2014, CD005019.
- Association of British Clinical Diabetologists and The Renal Association. Managing hyperglycaemia in people with diabetes and chronic kidney disease. London: ABCD, 2021.
Available at https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/Management-of-hyperglycaemia-in-adults%20-with-DKD.pdf (accessed 18th Jan 2022)
- Association of British Clinical Diabetologists and The Renal Association. Clinical practice guidelines for management of lipids in adults with diabetic kidney disease. London: ABCD, 2021.
Available at https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/Management-of-lipids-in%20adults-with-DKD.pdf (accessed 18th Jan 2022)
- Association of British Clinical Diabetologists and The Renal Association. Guidelines on the detection and management of diabetes post solid-organ transplantation. London: ABCD, 2021.
Available at https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/ABCD-RA-PTDM-v14.pdf (accessed 18th Jan 2022)
8. COMPLICATIONS OF CHRONIC KIDNEY DISEASE
Statements of Recommendation
We recommend that:
- Anaemia should be proactively sought in all transplant recipients; management requires accounting for immunosuppression in addition to other causes associated with progressive renal impairment. (1C)
- Mineral and bone disorders should be treated according to existing KDIGO and NICE guidelines. (1C)
We suggest that:
- Serum bicarbonate levels <22mmol/l should be treated by supplementing with oral sodium bicarbonate, escalating the dose according to the response. (2C)
Rationale
8.1 Introduction
Patients with failed allografts fare poorly according to standard measures of quality of CKD care, including anaemia management and phosphate control.1,2
8.2 Anaemia
Anaemia management should follow the pre-existing NICE and KDIGO guidelines for chronic anaemia in chronic kidney disease.3 Its correction may help extend graft life and improve patient quality of life.4 Successful management requires a distinct stepwise approach proactively identifying its presence, determining aetiology and then implementing appropriately targeted management.
8.2.1 Monitoring
Early detection can be achieved by reviewing trends in haemoglobin and haematinics. Target a haemoglobin range between 110-120 g/dl, avoiding drops <105 g/dl.
8.2.2 General Approach to Management
Consider causes of anaemia affecting the general population with CKD such as iron, folate, vitamin B12 and erythropoietin deficiency, or gastrointestinal loss; supplementation and correction are essential. The use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for hypertension in transplant recipients may contribute to the development of anaemia. In the early phases, transplant recipients may respond to oral iron however with progressive graft dysfunction intravenous iron is required to adequately replenish stores.
8.2.3 Specific Approach to Management
The impact of immunosuppression requires a distinct focus as it may contribute to anaemia and its identification. For instance, bone marrow suppression by anti-proliferative agents such as mycophenolate mofetil, azathioprine and leflunomide; microcytic anaemia associated with sirolimus;5,6 and haemolytic anaemia or aplastic anaemia associated with tacrolimus and cyclosporin in susceptible individuals.
Infections such as parvovirus-B19, BK polyomavirus, adenovirus and Epstein-Barr virus, which are more common in the transplant population, may also contribute to anaemia. Bacterial infections such as urinary sepsis can lead to a grumbling pro-inflammatory state which must be dealt with to enable adequate iron replacement and the expected response to erythropoietin. The possibility of lymphoproliferative disorder also requires consideration particularly as recipients may have had a significant time on immunosuppression.
The complex aetiology of anaemia in immunosuppressed transplant patients can lead to an increased incidence of resistant anaemia. It is not uncommon for the failing transplant recipient to be in a pro-inflammatory state due to chronic rejection. Recipients may require a higher body weight-adjusted erythropoietin dose relative to the general CKD population at the same GFR to achieve haemoglobin targets.
Anaemia management may require additional reduction of immunosuppression or changing agents. There may also be a case for consideration of relevant pharmacogenomics when initiating or changing immunosuppression, for instance, thiopurine S-methyltransferase (TPMT) status in relation to the use of azathioprine.7 Optimising the pharmacokinetics of mycophenolate exposure8,9 may also become more important as the transplant progresses through the failing stages.
8.3 Mineral and Bone Disorder
Bone and mineral disorders are common in KTRs and are associated with a high risk of fracture, morbidity and mortality. Pathophysiology is complex, involving pre-existing CKD-MBD and bone loss due to transplant-specific therapies, especially corticosteroids. However, our understanding is hampered by a paucity of available evidence in the form of bone biopsies, and a lack of well-designed prospective trials.10,11
We recommend that management encompasses the recommendations in the KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention and Treatment of CKD-MBD, Chapter 5: Evaluation and treatment of kidney transplant bone disease,12 alongside the NICE 2021 CKD: assessment and management guideline, 1.11 and 1.12.3
These can be summarised as:
8.3.1 Monitoring
Measure serum calcium, phosphate and parathyroid hormone concentrations in adults with a GFR of less than 30 ml/min/1.73 m2. Determine the subsequent frequency of testing by the measured values and the clinical circumstances.
8.3.2 Hyperphosphataemia management
Manage abnormalities as for patients with CKD stages 3-5.
8.3.3 Bone metabolism and osteoporosis
In patients with CKD stages 4–5T, we suggest that bone mineral density (BMD) testing not be performed routinely, because BMD does not predict fracture risk as it does in the general population and BMD does not predict the type of kidney transplant bone disease. In patients with CKD stages 4–5T with a known low BMD, we suggest management as for patients with CKD stages 4–5 not on dialysis.
8.3.4 Vitamin D supplementation
Manage as for patients with CKD stages 3-5.
8.4 Metabolic Acidosis
Metabolic acidosis (MA) is usually defined as a persistently low serum sodium bicarbonate level of less than 22mmol/l and is commonly observed in kidney transplant recipients with associated bone disease and muscle wasting. In children, MA impairs growth by inhibiting growth hormone secretion.
Recent studies have suggested it is an independent risk factor for CKD progression and ischemic cardiovascular events with associated graft failure and mortality and is positively associated with eGFR.
There is little data to associate the correction of MA with improved outcomes in kidney transplant recipients, but it would seem prudent to correct it according to patients with CKD.3,13
8.4.1 Monitoring
The frequency of monitoring of serum bicarbonate levels in kidney transplant recipients should be based on the clinical condition.
8.4.2 Treatment
We suggest treating bicarbonate levels <22mmol/l by supplementing with oral sodium bicarbonate 500mg three times a day, escalating the dose according to the response. Two serum sodium bicarbonate levels should be taken before starting treatment due to variations in serum level.14,15
Sodium citrate may cause less GI side effects but can be associated with aluminium toxicity. Novel agents are under investigation for future use.
Potentially sodium salts may worsen hypertension and fluid overload, but this is rarely seen in practice.
References
- Huml, A.M. and A.R. Sehgal, Hemodialysis Quality Metrics in the First Year Following a Failed Kidney Transplant. Am J Nephrol, 2019. 50(3): p. 161-167.
- Perl, J., et al., Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant, 2012. 27(12): p. 4464-72.
- National Institute for Health and Care Excellence (NICE) (2021) Chronic kidney disease: assessment and management. NG203. Available at: https://www.nice.org.uk/guidance/ng203.
- Jones, H., et al., Anemia after kidney transplantation; its prevalence, risk factors, and independent association with graft and patient survival: a time-varying analysis. Transplantation, 2012. 93(9): p. 923-8.
- Fishbane, S., et al., Posttransplant anemia: the role of sirolimus. Kidney Int, 2009. 76(4): p. 376-82.
- Maiorano, A., et al., Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation, 2006. 82(7): p. 908-12.
- Burnett, H.F., et al., Testing for thiopurine methyltransferase status for safe and effective thiopurine administration: a systematic review of clinical guidance documents. The Pharmacogenomics Journal, 2014. 14(6): p. 493-502.
- Kuypers, D.R., et al., Current target ranges of mycophenolic acid exposure and drug-related adverse events: a 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Ther, 2008. 30(4): p. 673-83.
- Cattaneo, D., et al., Pharmacokinetics help optimizing mycophenolate mofetil dosing in kidney transplant patients. Clin Transplant, 2001. 15(6): p. 402-9.
- Bouquegneau, A., et al., Bone Disease after Kidney Transplantation. Clinical Journal of the American Society of Nephrology, 2016. 11(7): p. 1282-1296.
- Vangala, C., et al., Mineral and Bone Disorders After Kidney Transplantation. Frontiers in Medicine, 2018. 5(211).
- KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011), 2017. 7(1): p. 1-59.
- Schulte, K., et al., Effect of Sodium Bicarbonate in Kidney Transplant Recipients With Chronic Metabolic Acidosis. Transplant Direct, 2019. 5(7): p. e464.
- Chen, W. and M.K. Abramowitz, Treatment of metabolic acidosis in patients with CKD. Am J Kidney Dis, 2014. 63(2): p. 311-7.
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter., Suppl. 2013; 3: 1–150.
9. MANAGEMENT OF THE COMPLICATIONS OF LONG-TERM IMMUNOSUPPRESSION
Although not specific to this group, many patients with a failing graft have been subject to long-term immunosuppression and we have covered some of the most common complications this treatment can cause.
9.1 Malignancy
9.1.1 Post-Transplant Lymphoproliferative Disorders (PTLD)
Statements of Recommendation
We recommend:
- All transplant recipients with PTLD receive joint care including a transplant physician and a specialist haematologist/haemato-oncologist. (1A)
- Sequential reduction in immunosuppression (RIS) with an initial withdrawal of anti-proliferative agent followed by a reduction of calcineurin inhibitor (CNI) dose by 30–50% whilst maintaining or reducing corticosteroids, with surveillance of graft function. (1B)
- Early disease response assessment (at 2–4 weeks) is recommended in those patients following RIS alone so that further treatment (chemotherapy) can be initiated in those that fail to respond. (1B)
- In the context of a failing kidney transplant, the objective of treatment should be to achieve complete remission of lymphoma with curative intent both to improve prognosis and to enable re-transplantation. (Ungraded)
- Re-transplantation can be considered after a minimum of 1-year disease-free period following remission, but a longer period may be needed in selected cases. (2C)
Recommendations for audit and research:
- Incidence of PTLD, histological subtypes, Epstein-Barr virus (EBV) and non-EBV associated lymphoma) in kidney transplant patients and characteristics including demographics, clinical presentation, management and outcomes.
- Re-transplantation rates and outcomes in patients with previous PTLD.
- The role of EBV-specific cytotoxic T-lymphocytes in the management of post-transplant lymphoma.
Rationale
Post-transplant lymphoproliferative disorders (PTLD) arise due to lymphoid or plasmacytoid proliferation in the context of immunosuppression (IS) following a solid organ transplant (SOT). PTLD accounts for 21% of all cancers in SOT recipients whereas lymphomas account for only 4-5% of all cancers in the immunocompetent population.1,3Although most cases are reported within one year of transplant, presentation beyond one year is not uncommon. Compared to other organs, kidney transplant recipients (KTR) have the lowest incidence of PTLD (0.8-2.5%).1,2The disease is usually EBV-driven diffuse large B cell lymphoma but Hodgkin’s lymphoma and non-EBV associated non-Hodgkin’s lymphoma are also described. These patients need multidisciplinary care, and it is recommended that all patients are discussed with a haemato-oncology multidisciplinary team (MDT) with input from transplant physicians. For details on diagnosis, staging, grading and management, please refer to British Society of Haematology guidelines.3
The management of PTLD usually involves a sequential reduction in IS. The anti-proliferative agent (azathioprine or mycophenolate mofetil) is withdrawn and the calcineurin inhibitor (CNI) dose is reduced by 30-50% whilst maintaining corticosteroids (prednisolone 5-10mg once daily). Most patients require chemotherapy including Rituximab, either as a single agent or in combination with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone). Mild, localised disease may respond to IS reduction alone and initial close observation. In high-risk cases with more advanced and aggressive disease (Ann Arbor stage ≥III, elevated LDH and more than one extra nodal disease), concomitant chemotherapy would be required along with RIS. A significant reduction or withdrawal of IS carries a risk of rejection and graft failure which is easier to manage for KTR than heart or lung transplant recipients where, unlike kidney failure, long term organ support therapies are not available. In kidney transplant patients, the patient and graft specific factors to take into consideration while reducing/stopping immunosuppressive medications include graft function and urine output.
In the context of a failing kidney transplant, the management of PTLD should focus on achieving complete remission of lymphoma with curative intent both to improve patient prognosis and to enable re-transplantation. This usually means a more aggressive reduction or complete withdrawal of immunosuppression depending on the severity and extent of lymphoma followed by chemotherapy. Transplant nephrectomy should be considered in patients with poor graft function where the kidney is infiltrated by lymphoma.
With regards to retransplantation, the current joint guideline of the British Committee for standards in Haematology and the British Transplant Society recommends that patients with PTLD wait for at least one year after achieving complete metabolic remission prior to consideration of retransplantation.3 It is advisable to have undetectable or low EBV viral load prior to retransplantation as increased IS in the early post-transplant period can lead to an increase in viremia and consequent increased risk of B cell proliferation and development of lymphoma. However, due to a lack of evidence in this field, we are unable to recommend a threshold blood EBV level (viral load) prior to transplantation and there are reports of re-transplantation in patients with active EBV viremia.4 The risk-to-benefit ratio of proceeding with kidney transplantation in patients with previous PTLD and ongoing EBV viremia should be considered carefully on a case-by-case basis and decisions made in liaison with the haemato-oncologist.
For relapsed or refractory EBV positive B cell PTLD, adoptive immunotherapy with EBV -specific cytotoxic T cells (CTLs) offers another therapeutic option without the risk of graft rejection. These EBV-specific cytotoxic T cells are either the recipient’s own T cells used to generate EBV-directed T cells (autologous) or from a bank of HLA-matched/partially matched EBV-specific T cells.5,6,7 Although this treatment is currently not widely used, increasing safety and efficacy data could make it more available in the future.
References
- Caillard S, Porcher R, Provot F, et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol 2013;31:1302-9.
- Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 2004;4:222-30.
- Shah N, Eyre TA, Tucker D, et al. Front-line management of post-transplantation lymphoproliferative disorder in adult solid organ recipient patients – A British Society for Haematology Guideline. Br J Haematol 2021;193:727-40.
- Caillard S, Cellot E, Dantal J, et al. A French Cohort Study of Kidney Retransplantation after Post-Transplant Lymphoproliferative Disorders. Clin J Am Soc Nephrol 2017;12:1663-70.
- Savoldo B, Goss JA, Hammer MM, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood 2006;108:2942-9.
- Vickers MA, Wilkie GM, Robinson N, et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br J Haematol 2014;167:402-10.
- Liu JY, Zhang JM, Zhan HS, Sun LY, Wei L. EBV-specific cytotoxic T lymphocytes for refractory EBV-associated post-transplant lymphoproliferative disorder in solid organ transplant recipients: a systematic review. Transpl Int. 2021; 34(12):2483-2493.
9.1.2 Solid Organ Cancers
Statements of Recommendation
We recommend that:
- Screening for cancer in patients with a failing kidney transplant should be done in accordance with national guidelines (for cervical, breast and colon cancer). Screening for renal cell cancer is not recommended. (2C)
- Patients should be aware of cancer risk and encouraged to report symptoms which may represent malignancy (breast lump, testicular lump, lower urinary tract symptoms, haematuria). (2D)
- Kidney transplant recipients with a failing graft and previously treated cancer should be considered for re-transplantation. (1B)
- Recommended time interval between remission from cancer and transplantation depends on the type, grade and stage of the cancer. (Ungraded)
Recommendations for audit and research:
- Outcomes of patients re-transplanted following treatment of solid organ cancer.
- Optimal IS regimens for kidney transplant recipients with solid organ cancers.
Rationale
Kidney transplant recipients are at higher risk of developing certain solid organ cancers, thought to be in part mediated by long-term IS and altered T cell anti-tumour immune surveillance. The risk varies for different cancers (summarised in the RA/BTS Clinical Practice Guideline Post-operative care in kidney transplant recipients1). The main objective of the management of transplant recipients with cancer is to achieve complete remission/cure from cancer with surgery, chemotherapy, radiotherapy and immunotherapy as required. Preservation of renal function facilitates adequate cancer treatment and improves tolerance to chemotherapy. Therefore, preserving renal function and residual urine output with continuation of low-level IS should be considered while the patient undergoes active treatment for cancer under surgical and oncology teams. Complete withdrawal of IS can lead to severe rejection with consequent hyperinflammatory state, anaemia and return to dialysis which is an undesirable outcome for patients undergoing treatment for cancer. Liaison with the oncology team is recommended when considering any augmentation of IS.
Patients with a failing kidney transplant and a history of successfully treated cancer can be considered for kidney re-transplantation. However, it is important to estimate the risk of cancer recurrence and the patient needs to be counselled accordingly before being placed on the deceased donor waiting list for a second or subsequent transplant or proceeding with living donor transplantation. Solid organ cancer recurrence is dependent on tumour type and the time interval between remission/cure and transplantation with a longer interval associated with a lower risk of recurrence. Overall, 53% of recurrences occur in patients transplanted within 2 years of cancer treatment, falling to 34% if the interval is 2-5 years and 13% if the interval is > 5 years but this is dependent on the organ involved, stage and histological grade at the time of diagnosis and the response to treatment. 2,3
Appendix 9.1 recommends the waiting time following curative treatment of malignancy prior to proceeding to re-transplantation, adapted from a recent American consensus expert opinion.2 It is essential to make decisions following discussions with the treating Oncologist and Surgeon. Most recurrences occur within 3 years; generally, once a disease-free interval of 3–5 years after all treatments has been completed, patients could be considered as transplant candidates.
References
- Renal association and British Transplantation Society clinical practice guideline on post-operative care in the kidney transplant recipient. Richard J Baker, Patrick B Mark, Rajan K Patel, Kate K Stevens, Nicholas Palmer. BMC Nephrol. 2017; 18: 174 (https://bts.org.uk/wp-content/uploads/2017/06/FINAL_PostOperative_Care_Guideline.pdf)
- Al-Adra DP, Hammel L, Roberts J, et al. Pretransplant solid organ malignancy and organ transplant candidacy: A consensus expert opinion statement. Am J Transplant 2021;21:460-74.
- Acuna SA, Sutradhar R, Kim SJ, Baxter NN. Solid Organ Transplantation in Patients With Pre-existing Malignancies in Remission: A Propensity Score Matched Cohort Study. Transplantation 2018;102:1156-64.
Appendix 9.1
Recommended wait time for transplant candidates with prior history of:
Breast cancer
| Risk/Stage | Recommended time interval to transplant | Additional considerations |
| Low risk: Ductal carcinoma in situ and stage I | No wait time | Continuation of endocrine therapy is not a contraindication for transplantation. Oestrogen receptor-negative disease may have a slightly higher rate of recurrence in the first 2-3 years |
| Intermediate Risk
Stage II |
2 Years | Oestrogen receptor-negative disease may have slightly higher rate of recurrence in first 2-3 years |
| High risk
Stage III |
3-5 Years | Oestrogen receptor-negative disease may have slightly higher rate of recurrence in first 2-3 years.
Inflammatory breast cancer likely has a higher risk of recurrence and worse survival |
Colon cancer
| Risk/Stage | Recommended time interval to transplant | Additional considerations |
| Low risk
Stage I (T1 or T2, N0, M0) |
1 Year | High-risk features:
Poorly differentiated histology, bowel obstruction, tumour perforation |
| Low Intermediate risk
Stage II (T3, N0, M0) |
2 Years, longer if high-risk features present | |
| High Intermediate risk
Stage II (T4, N0, M0) Stage III (Any T, N+, M0) |
3 years, 5 years if high-risk features present | |
| High risk
Stage IV (Any T, Any N, M+) |
5 years
In patients with residual disease/metastasis, transplantation is not recommended |
Rectal cancer
| Risk/Stage | Recommended time interval to transplant | Additional considerations |
| Low risk
Stage I (T1 or T2, N0, M0) |
1 year, consider 2 years if high risk features are present | Poorly differentiated histology, bowel obstruction, tumour perforation, lower 1/3rd of rectum |
| High intermediate risk
Stage II (T3, T4, N0, M0) Stage III (Any T, N+, M0) |
3 years, 5 years if high-risk features are present | |
| High Risk
Stage IV (Any T, Any N, M+) |
5 years. In patients with residual disease/metastasis, transplantation is not recommended |
Urological malignancies
Prostate Cancer
| Risk/Stage | Recommended time interval to transplant | Additional considerations |
| Very Low Risk and low risk
-PSA<10ng/mL -3 or fewer cores of Gleason 6 (grade group 1); no greater than 50% of individual core -T1c-T2a |
None | Surveillance recommended post-transplant |
| Low- volume
Intermediate risk One of the following criteria: -PSA >10ng/mL, Gleason 7 (grade group 2 or 3), T2b |
If decision is surveillance only, no wait time
If treatment is initiated and nomogram (www.nomograms.org) predicts cancer-specific death over the next 15 years <10%, no wait time |
Post-transplant, surveillance and treatment if required depending on patient and cancer characteristics |
| High-Volume Intermediate risk, High Risk or Very High Risk
-PSA>20ng/mL or High volume Gleason 7 or any Gleason 8-10, T3 |
If treated, deemed cured and nomogram predicts cancer-specific death over the next 15 years <10%, no further wait time | May require treatment post-transplant if there is disease recurrence |
| Metastatic disease | Transplantation is not recommended | Castration-sensitive metastatic disease, treated with local and systemic therapy, stable disease for 2 years, may consider transplant |
Renal cell carcinoma
| Risk/Stage | Recommended time interval to transplant | Additional considerations |
| T1a (≤4 cm), N0, M0 | No wait time | Resection/nephrectomy before listing is the standard approach but in selected cases, active surveillance can be an option. A biopsy can be useful in guiding management. |
| T1b (>4cm ≤7cm), N0, M0 | 1-2 years based on histology (Fuhrman grade) | Fuhrman grade (FG)1 -2: consider no wait time, FG 3-4: 1-2 years |
| T2 (7-10 cm), N0, M0 | 2 Years | |
| T3, N0, M0 | 2-3 years | |
| T4, N0, M0 | 2-3 years | |
| Any T, Node positive, metastatic disease | Not a candidate | If solitary metastasis is resected and disease free for 2-3 years, MDM discussion |
Bladder cancer
| Risk/Stage | Recommended time interval to transplant | Additional considerations |
| Non-muscle invasive bladder cancer, low and Intermediate risk | 6 months | |
| Non-muscle invasive bladder cancer, High Risk | 2 years | |
| Muscle invasive, post radical cystectomy | 2 Years | |
| Muscle invasive, post chemo and radiotherapy | Not a transplant candidate |
Gynaecological cancer
| 5-Year recurrence risk | Type and stage | Recommended time interval to transplant |
| Low risk (<5% risk of recurrence) | Stage I/IB, grade 1-2 endometrial cancer without lymph-vascular space invasion | No waiting period after the completion of primary treatment |
| Stage IA/IB/IC Grade 1-2 ovarian cancer | ||
| Stage IA1, IA2 squamous/ adenocarcinoma of the cervix | ||
| Intermediate Risk
5-15% risk of recurrence |
Stage II endometrial cancer | 2-3 years after completion of treatment |
| Stage IB squamous/adenocarcinoma of the cervix | ||
| High Risk
>30% risk of recurrence |
Endometrial cancer: Aggressive/high-grade histology, any stage
Stage III endometrial cancer |
5 years after completion of treatment
5 years after completion of treatment |
| Stage II/III Ovarian cancer | ||
| Stage II/III Squamous cell/adenocarcinoma of cervix | ||
| Very high risk | Stage IV endometrial cancer (all grades)
Recurrent or metastatic endometrial cancer |
Not a transplant candidate |
| Stage IV Ovarian cancer (any grade)
Recurrent or metastatic Ovarian Cancer |
||
| Stage VI Cervical cancer
Recurrent or metastatic cervical cancer |
Lung cancer
| Stage | Recommended wait time |
| I (T1a, 1b, N0) | ≥3 years |
| I (T1c N0) | 3-5 years |
| IB (T2aN0) | 5 years |
| IIA (T2bN0) | 5 years |
| IIB (T3N0) | 5 years |
| IIIA | 5 years |
| Stage IIIB and higher | Not a transplant candidate |
9.1.3 Skin cancers
The recommendations that are in place in the BTS/RA Clinical Practice Guideline ‘Post-operative care in the kidney transplant recipient’ should be continued for the management of skin disorders in the failing graft.6
Statements of Recommendation
We recommend that:
- Renal transplant recipients should be educated about the adverse effects of sun exposure. (1C)
- Patients should be encouraged to cover their skin in direct sunlight and to use total sunblock (Sun Protection Factor ≥50). (1D)
- Self-examination should be encouraged with guidance provided. This should be supplemented by a biannual review by a trained healthcare professional up to 5 years post-transplant and an annual review from 5 years. (2C)
- After initial screening, the frequency of subsequent reviews can be guided by a dermatologist based on risk assessment (skin type, previous history of skin cancers, immunosuppressive burden and any premalignant lesions). (Ungraded)
- Prescription of acitretin as chemoprophylaxis to be considered in those with ≥2 previous non-melanoma skin cancers (NMSC) if there are no contraindications. (2B)
- IS should be reduced if neoplasia develops. (2C)
- Following re-transplantation, CNI to mTOR inhibitor (Sirolimus or Everolimus) switch should be considered for recipients with a history of skin cancers, particularly squamous cell skin cancer. (2C)
- No waiting period before re-transplantation is required for basal or low-risk squamous cell carcinoma of the skin (surgical excision with clear margins), in situ melanoma following curative wide local excision. (1B)
- Following surgical excision with clear margins, high-risk squamous cell carcinoma of the skin without perineural invasion requires a two-year waiting period before re-transplantation. If the perineural invasion is found or adjuvant radiation therapy is required, a two- to three-year waiting period is required. (1B)
- Localised Merkel cell carcinoma of the skin treated with wide local excision requires a two-year, cancer-free period before re-transplantation (1B)
- A stage Ia melanoma treated with wide local excision requires a two-year, disease-free period. Other more advanced melanoma may require a five-year waiting period or maybe a contraindication to re-transplantation. (1B)
Recommendations for audit and research:
- Recurrence of skin cancers following re-transplantation in patients with a previous history of treated skin cancers
- Optimal immunosuppressive regimen in patients with a history of skin cancers following re-transplantation
Rationale
The incidence of cutaneous neoplasia is significantly increased in kidney transplant recipients compared to the general population.1,2,3,4 The relative risk of developing melanoma and non-melanoma skin cancers are summarised in the BTS guideline on post-operative care of kidney transplant recipient.6 Patients with a failing kidney transplant are at higher risk of skin cancers due to a longer duration of immunosuppression exposure.5 A variety of factors, including the intensity and duration of IS, the patient’s ethnic background, sun exposure history, and geographic location, influence the likelihood of the development of skin cancer in these patients.
Regardless of graft function, the mainstay of management is the same with regular surveillance, reduction in IS and specific management by the dermatology/surgical team. Part of the treatment will involve the minimisation or modification of immunosuppressive therapy. After re-transplantation, a switch to mTORi should be considered in patients with previously treated skin cancers, particularly squamous cell skin cancer. 12, 13
The recommended twice-a-year review by a dermatologist for 5 years followed by an annual review is a target that is often not met in most centres. A survey published in 2020 found wide variations in skin surveillance for kidney transplant patients in the UK. Of the 51 (86%) centres responding, only 28 (55%) provided skin cancer surveillance post-transplantation.10,11 There are wide variations in the frequency of monitoring and a recommended biannual assessment is often not achieved. Patients with a failing kidney transplant would have been exposed to immunosuppression for prolonged periods of time and therefore, it is advisable to ensure annual skin surveillance or at a frequency determined by the dermatologist. Following second and subsequent transplants, at least one dermatology review within the 1st post-transplant year, followed by a review at intervals advised by the dermatology team (based on the previous history of skin cancers, skin type and current skin assessment) where bi-annual assessment is not possible could be a practical approach to achieve adequate dermatology surveillance. Patients should be encouraged to inspect their skin and report any new lesions.
The role of specific immunosuppressants on skin malignancies is unclear although limited evidence suggests that mycophenolic acid compounds may be less likely to cause NMSC compared to azathioprine. mTOR inhibitors may reduce the risk for NMSC in renal transplant patients compared with other immunosuppressive regimens.12 The use of these agents should be carefully balanced with adverse effects and impact on graft function on a case-by-case basis.
References
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003 Apr 24;348(17):1681-91. doi: 10.1056/NEJMra022137. PMID: 12711744.
- Greenberg JN, Zwald FO. Management of Skin Cancer in Solid-organ Transplant Recipients: A Multidisciplinary Approach. Dermatol Clin. 2011 Apr;29(2):231-41, ix. doi: 10.1016/j.det.2011.02.004. PMID: 21421148.
- Chen QP, Aw DC. Epidemiology of skin diseases in renal transplant recipients in a tertiary hospital. Ann Acad Med Singap. 2010 Dec;39(12):904-5. PMID: 21274486.
- Garrido PM, Borges-Costa J. Skin disorders in renal transplant recipients: a retrospective study. An Bras Dermatol. 2017;92(5):638-41.
- British Transplantation Society. Management of the Failing Kidney Transplant. Available at:https://btsorguk/wp-content/uploads/2016/09/13_BTS_Failing_Graft-1pdf
- Baker R J et al, Post operative care in the kidney transplant recipient, British Transplantation Society, February 2017
- Garg S, Carroll RP, Walker RG, Ramsay HM, Harden PN. Skin cancer surveillance in renal transplant recipients: re-evaluation of U.K. practice and comparison with Australian experience. Br J Dermatol 2009;160:177-9.
- Ramsay HM, Reece SM, Fryer AA, Smith AG, Harden PN. Seven-year prospective study nonmelanoma skin cancer incidence in U.K. renal transplant recipients. Transplantation 2007;84: 437-439
- Zwald F, Leitenberger J, Zeitouni N, Soon S, Brewer J, Arron S, Bordeaux J, Chung C, Abdelmalek M, Billingsley E, Vidimos A, Stasko T. Recommendations for Solid Organ Transplantation for Transplant Candidates With a Pretransplant Diagnosis of Cutaneous Squamous Cell Carcinoma, Merkel Cell Carcinoma and Melanoma: A Consensus Opinion From the International Transplant Skin Cancer Collaborative (ITSCC). Am J Transplant. 2016 Feb;16(2):407-13. doi: 10.1111/ajt.13593. Epub 2016 Jan 28. PMID: 26820755.
- https://www.kidneycareuk.org/news-and-campaigns/news/kidney-clinic-skin-deep-skin-cancer-aftertransplant
- Skin cancer screening in organ transplant centres in the United Kingdom: A national survey. Andrea Cordaro, Thomas D. Dobbs, John A. G. Gibson, Sairan Whitaker& Iain S. Whitaker. European Journal of Dermatology volume 30, pages372–376 (2020)
- Robert P Carroll, Joanna Hester, Kathryn J Wood, Paul N Harden. Conversion to sirolimus in kidney transplant recipients with squamous cell cancer and changes in immune phenotype. Nephrol Dialy Transplant 2013 Feb;28(2): 462-465
- Salgo R, Gossmann J, Schofer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-) malignancies and non-melanoma skin cancer in a prospective, randomised, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10:1385-93
9.2 BK Virus and Nephropathy
Statements of Recommendation
We recommend that:
- Re-transplantation can be safely considered in patients who develop BK viraemia or nephropathy in an earlier graft. (2C)
- Clearance of viraemia is desirable prior to re-transplantation. (2C)
Recommendations for audit and research:
- Prevalence and long-term outcomes of BK viraemia and BK nephropathy (BKVN) in renal transplant patients and following re-transplantation.
Rationale
The BK polyoma virus infection can cause viraemia, BKV nephropathy and transplant ureteric stenosis in the kidney transplant patient. The reported prevalence is up to 10% with 15-20% of patients losing their allograft due to BKV nephropathy.1 Reduction in immunosuppression is the mainstay of BKVN treatment. Usual practice is withdrawal of antiproliferative agents such as mycophenolate mofetil and azathioprine and maintaining tacrolimus levels between 4-6µg/L (or Ciclosporin A 50-100 µg/L) with or without prednisolone but evidence supporting this is limited.2,3,4Specific agents such as quinolones, leflunomide and cidofovir have been used to treat BKV nephropathy with limited evidence.2 Leflunomide, an immunosuppressive agent with antiviral activity, is often a preferred choice due to better tolerability with no nephrotoxicity.5
In the context of the failing renal allograft, the indications for screening and treatment strategy largely remain the same. There is no evidence that allograft loss due to BKN adversely affects the outcome of a subsequent graft.7Clearance of viraemia is recommended prior to re-transplantation2, but the presence of a low-grade viraemia despite appropriate reductions or withdrawal of immunosuppression in the context of failing transplant kidney is not an absolute contra-indication for re-transplantation.6 Currently, there is not enough evidence to support graft nephrectomy to eliminate a potential reservoir of BKV and a source of future infection prior to re-transplantation.
References
- Renal association and British Transplantation Society clinical practice guideline on post-operative care in the kidney transplant recipient. Richard J Baker, Patrick B Mark, Rajan K Patel, Kate K Stevens, Nicholas Palmer. BMC Nephrol. 2017; 18: 174
- BK virus infection: an update on diagnosis and treatment. Deirdre Sawinski, Simin Goral. Nephrol Dial Transplant. 2015;30: 209-217
- Weiss AS, Gralla J, Chan L, et al. Aggressive immunosuppression minimisation reduces graft loss following diagnosis of BK virus-associated nephropathy: a comparison of two reduction strategies. Clin J Am Soc Nephrol. 2008; 3: 1812-1819
- Hardinger KL, Koch MJ, Bohl DJ et al. BK virus and the impact of pre-emptive immunosuppression reduction: 5 year results. Am J Transplant. 2010; 10: 407-415
- Williams JW, Javaid B, Kadambi PV, et al. Leflunomide for polyomavirus type BVK nephropathy. N Engl J Med 2005; 352: 1157-1158
- Geetha D, Sozio SM, Ghanta M, Josephson M, Shapiro R, Dadhania D, Hariharan S. Results of repeat renal transplantation after graft loss from BK virus nephropathy. Transplantation. 2011 Oct 15;92(7):781-6. doi: 10.1097/TP.0b013e31822d08c1. PMID: 21836535.
- Dharnidharka VR, Cherikh WS, Neff R, et al. Re transplantation after BK virus nephropathy in prior kidney transplant: an OPTN database analysis. Am J Transplant. 2010; 10: 1312-1315
9.3 Recurrent Primary Disease
Statements of Recommendation
We recommend:
- Re-transplantation should be considered for patients with a failing kidney transplant due to recurrent glomerulonephritis despite the potential for recurrent disease. (1D)
- Prior to re-transplantation, appropriate counselling should be given to patients with conditions known to recur quickly and severely in a kidney transplant, namely FSGS, atypical HUS and other complement abnormalities (C3GN and Dense Deposit Disease). (1D)
- Patients with recurrent FSGS in the transplanted kidney should be considered for treatment with plasma exchange and rituximab. (ungraded)
- Prophylactic plasma exchange and rituximab for patients with FSGS to prevent recurrence are not recommended. (1D)
- Patients with MCGN due to complement abnormalities (C3GN and Dense Deposit Disease) should be discussed in a regional multidisciplinary meeting pre-transplantation to determine a plan for recurrent disease. (ungraded)
- Patients with atypical HUS with defined complement abnormalities and C3GN should be discussed with the UK national atypical HUS service before transplantation to have a clear management plan in case of disease recurrence post-transplant. (1C)
- Eculizumab may be helpful in reducing the risk of recurrent C3GN and atypical HUS. (ungraded)
- If advanced interstitial fibrosis and tubular atrophy are present on kidney biopsy, specific treatments for recurrent disease are not recommended. (2D)
- Patients with recurrent glomerulonephritis and proteinuria should be treated with RAS blockade to control proteinuria and hypertension. (1C)
Recommendations for audit and research:
- Studies to determine the optimum dosing schedule for rituximab and plasmapheresis for FSGS post-recurrence.
- Re-transplantation rates and outcomes for patients with graft failure due to recurrent atypical HUS or complement defect associated with MCGN.
- The safety and efficacy of SGLT2 inhibitors in patients with recurrent proteinuric disease in the kidney transplant.
Rationale
Recurrent primary glomerular disease in the transplanted kidney is a common histological occurrence but does not inevitably lead to accelerated graft failure. For example, 50% of patients with diabetes mellitus pre-transplant show mesangial expansion on renal transplant biopsy 5 years post-transplant.1 Renal Registry data from the UK indicates around 3.5% of kidney transplants fail due to recurrent disease.2 However, other studies quote a higher rate, with 18-22% graft losses due to recurrent or de novo glomerulonephritis.3 Histological patterns in a failing transplant kidney are often driven by more than one pathology, including chronic rejection (T cell and antibody-mediated), chronic CNI toxicity, hypertension, vascular disease, diabetes, and recurrent glomerular disease. Consequently, it is often difficult to attribute graft failure to recurrent disease alone.4 Recurrent FSGS, C3 Glomerulopathy (subdivided into dense deposit disease, formerly Type2 mesangiocapillary glomerulonephritis and C3 glomerulonephritis), membranous nephropathy and IgA nephropathy all increase the risk of allograft loss.5 FSGS and C3G typically recur in the early post-transplant period whereas the risk of recurrence of other forms of glomerulonephritis increases with time post-transplantation.6 Although disease recurrence may shorten graft survival, long-term outcome data support transplantation (both deceased and living donor) in patients with primary glomerulonephritis.7Renal registry data from Australia and New Zealand, collected over a 28-year follow-up period, has demonstrated the recurrence rates for primary glomerulonephritis after first and subsequent transplants (table 1).
Table 1. Recurrence rates for primary glomerulonephritis
| FSGS n (%) (n = 975) | IGAN n (%) (n = 2393) | MCGN n (%) (n = 348) | MN n (%) (n = 309) | Other GN n (%) (n = 3211) | |
| Total | 101 (10.4%) | 231 (9.7%) | 54 (15.5%) | 38 (12.3%) | 87 (2.7%) |
| First graft | 79 of 861 (9.1) | 210 of 2162 (9.7) | 48 of 288 (16.6) | 38 of 278 (13.6) | 76 of 2886 (2.6) |
| Second graft | 19 of 98 (19.3) | 20 of 215 (9.3) | 6 of 55 (10.9) | 0 of 30 (0) | 10 of 288 (3.4) |
| Subsequent grafts | 3 of 16 (18.7) | 1 of 16 (6.2) | 0 of 5 (0) | 0 of 1 (0) | 1 of 37 (2.7) |
Recurrent FSGS in the first kidney transplant increases the risk of recurrent FSGS in a subsequent transplant/s with a shorter duration from diagnosis to kidney failure often associated with a higher risk of early recurrence post-transplant. Some studies have suggested this risk is upwards of 60-80%.8
Other important causes of recurrent diseases include primary oxalosis, atypical haemolytic uraemic syndrome, C3G and Fabry disease.
Appropriate counselling with specific reference to high rates of recurrence and graft failure (in those with aggressive recurrence) in patients with FSGS is essential pre-transplant and should be part of donor counselling for living donor kidney transplants. Patients with atypical HUS and C3G are at high risk of recurrence in transplant kidney9and should also receive appropriate counselling pre-transplant with a clear management plan discussed and decided pre-transplant at a multidisciplinary meeting.
Early recurrence with nephrotic range proteinuria and rapidly declining kidney function can occur in recurrent FSGS (with up to 60% graft loss) and this merits consideration of aggressive management options that include plasmapheresis and Rituximab.10 The usual regime used is 5 sessions of plasmapheresis followed by 2 doses of Rituximab of 1g each given 2 weeks apart. Some patients require repeat sessions of plasmapheresis and Rituximab depending on their clinical course. A prophylactic strategy to give plasmapheresis and Rituximab for prevention of FSGS recurrence is not recommended.11 Patients with atypical HUS with defined complement abnormalities and MCGN with associated complement abnormalities (C3 glomerulonephritis and dense deposit disease) should be discussed at regional transplant multidisciplinary meetings and UK national atypical HUS service before transplantation to have a clear management plan in case of disease recurrence post-transplant. Selective complement inhibition with Eculizumab has been shown to be an effective preventive strategy for patients at high risk of recurrence (based on the clinical history and complement defects) and for the management of recurrent disease.12 However, given the high cost of this therapy and uncertainties around the duration of therapy post-transplant, it is important to establish a clear management plan pre-transplant.
No specific recommendation can be made for the management of recurrent IgA nephropathy apart from the general management of hypertension and proteinuria with renin-angiotensin system (RAS) blockade. Emerging new therapies for IgA disease could potentially change this.
Other diseases that can rarely recur in the transplanted kidney include membranous nephropathy, ANCA-associated vasculitis and SLE. Specific treatment similar to that used for the disease in the native kidney may be indicated. Persistent seropositivity for disease-associated autoantibodies (eg, anti-PLA2R or ANCA) is not a contra-indication to re-transplantation in patients in clinical remission. Management of these patients will need to be decided on a case-by-case basis.
In the context of a failing transplant kidney with progressive decline in eGFR and advanced tubulointerstitial fibrosis on biopsy, specific treatment targeted to recurrent glomerular disease is not recommended. The management in this situation should be focused on blood pressure control, optimisation of diabetes treatment, attention to other cardiovascular risk factors (smoking and lipids), weight management and management of complications associated with CKD (anaemia and bone disease). Their care may be best coordinated in a low-clearance transplant clinic.
References
- Coemans, M., et al., Occurrence of Diabetic Nephropathy After Renal Transplantation Despite Intensive Glycemic Control: An Observational Cohort Study. Diabetes Care, 2019. 42(4): p. 625-634.
- Burton, H., et al., Causes of renal allograft failure in the UK: trends in UK Renal Registry and National Health Service Blood and Transplant data from 2000 to 2013. Nephrology Dialysis Transplantation, 2018. 34(2): p. 355-364.
- Cosio, F.G. and D.C. Cattran, Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int, 2017. 91(2): p. 304-314.
- Naesens, M., et al., The histology of kidney transplant failure: a long-term follow-up study. Transplantation, 2014. 98(4): p. 427-35.
- Jiang, S.H., A.L. Kennard, and G.D. Walters, Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol, 2018. 19(1): p. 344.
- Sellarés, J., et al., Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant, 2012. 12(2): p. 388-99.
- Pippias, M., et al., Long-term Kidney Transplant Outcomes in Primary Glomerulonephritis: Analysis From the ERA-EDTA Registry. Transplantation, 2016. 100(9): p. 1955-62.
- Artero, M., et al., Recurrent focal glomerulosclerosis: natural history and response to therapy. Am J Med, 1992. 92(4): p. 375-83.
- Regunathan-Shenk, R., et al., Kidney Transplantation in C3 Glomerulopathy: A Case Series. Am J Kidney Dis, 2019. 73(3): p. 316-323.
- Alasfar, S., et al., Rituximab and Therapeutic Plasma Exchange in Recurrent Focal Segmental Glomerulosclerosis Postkidney Transplantation. Transplantation, 2018. 102(3): p. e115-e120.
- Boonpheng, B., et al., Rituximab or plasmapheresis for prevention of recurrent focal segmental glomerulosclerosis after kidney transplantation: A systematic review and meta-analysis. World J Transplant, 2021. 11(7): p. 303-319.
- Zuber, J., et al., Use of Highly Individualized Complement Blockade Has Revolutionized Clinical Outcomes after Kidney Transplantation and Renal Epidemiology of Atypical Hemolytic Uremic Syndrome. J Am Soc Nephrol, 2019. 30(12): p. 2449-2463.
10. PATIENT INVOLVEMENT AND OPTIONS FOR RENAL REPLACEMENT THERAPY
Statements of recommendation
We recommend that:
- Patients with a failing transplant should have the opportunity to discuss different modalities of renal replacement therapy (RRT) including re-transplantation, haemodialysis, peritoneal dialysis and conservative care. This discussion may involve members of their core support group. (1C)
- All patients should be considered for re-transplantation if there is no absolute contraindication when they have an eGFR <20mls/min/1.73m2or if there is an expectation their graft will fail within the next 12 months. (1C)
- We recommend pre-emptive re-transplantation in suitable candidates as the best option, ideally occurring when their eGFR is 10-15 mL/min/1.73m2. (1C)
We suggest that:
- An initial directed discussion by the transplant consultant to take place with the patient and their core support group about their failing transplant, as this is important in preparing them for future change. (ungraded)
- Where sufficient numbers or resources exist, care for people with a failing transplant should occur in a dedicated failing transplant clinic. (ungraded)
- Offer peer support/buddy or mentor services with expert patients which may support decision-making. (ungraded)
- The timing of dialysis initiation should be based on clinical factors and symptoms related to chronic kidney disease (CKD) progression rather than eGFR. (ungraded)
Recommendations for research:
- Development of tools to assist in the prediction of transplant failure timing based on patient factors, clinical observations, biomarkers etc.
- Comparative data on patient-level outcomes for those with failed allografts undergoing retransplantation, returning to dialysis and conservative management to inform shared decision-making.
- Examination of the potential implications of dedicated failing transplant clinics on patient level outcomes.
Rationale
10.1 Introduction
While there are some important transplant-specific issues, the management of end stage kidney disease (ESKD) in the context of a failing transplant is largely the same as for patients with CKD. Please see the UKKA Guideline ‘Planning, Initiating and Withdrawal of Renal Replacement Therapy’.1 Decision-making is also similar to patients with CKD. People with failing transplants may be unfamiliar with current dialysis practices and may need updating regarding current treatment modalities.2 This is often neglected or delayed when compared to the general CKD population.3 Several means of sharing and discussing information can be considered, depending on local availability:
- Shared decision-making information regarding dialysis treatment by a specialist renal nurse – individually and in a group setting.
- Use of decision aids to improve knowledge and patient participation.4, 5
- Providing written and visual information for reference at home and to share with their core support group.
Patient information leaflets are available from the kidney patient support charity, Kidney Care UK on a wide range of topics relating to CKD, renal replacement therapy, dialysis access and conservative management.
The time course of kidney transplant function decline is often less predictable compared to the decline of native kidney function, emphasising the importance of early decision-making.6 We suggest referral to a dedicated failing transplant clinic or return to a local advanced kidney care clinic to facilitate contact with all members of the MDT and ensure a smooth transition to the next modality of RRT. The location will depend on the local resources, staff availability and patient choice. If this involves returning to a local centre for dialysis or conservative management, we recommend transferring care at least 6 – 12 months before allograft failure is anticipated to ensure sufficient time for preparation.
The ‘Transplant Second’ mindset is essential to identify candidates in a timely fashion and to ensure appropriate pre-emptive re-listing. This has important benefits for the patient, their core support group and society.
10.2 Patient involvement
At all stages, shared decision making between the patient, their core support group and renal MDT should be encouraged.7 This approach can promote patient motivation and preparedness which is important at the time of potential psychological upheaval when transitioning from protecting remaining renal function to future planning.8, 9Patients may be adversely affected by their emotions and this can pose a barrier to timely planning2, 10 (see Section 4 Psychosocial Issues).
10.3 Physical, social and mental wellbeing
10.3.1 General Principles
The individual should be as mentally and physically able to commit to the transition process from transplantation to re-transplantation, dialysis or conservative management/ palliative care with the minimum social impact to the individual and their core support group.
10.3.1.1 Mental, Emotional & Social Wellbeing
We suggest patients have a psychosocial needs assessment when first identified to have a failing transplant and at the time of transition to an alternative RRT. This should be followed-up with a referral to appropriate services where required. Under-detection of emotional distress if not addressed can adversely affect medical management and lead to greater illness progression.11
Referral to Renal Psychosocial Practitioners can be made based on Patient Health Questionnaire (PHQ4) 12, Distress Thermometer (DT) 13, Work and Social Adjustment Scale (WSAS) 14 or Medication Adherence Report Scale 5 (MARS 5) 15 (see also Section 4, Psychosocial Issues).
10.3.1.2 Physical Wellbeing
An evaluation of physical activity levels at the time of re-listing is reported to be a valuable tool for risk stratification and prediction of post-transplant patient survival.16 Recommendations for physical activity should consider age, ethnic background, presence of other comorbidities and access to resources. For patients at higher risk of falls, healthcare providers should provide advice on the intensity of physical activity (low, moderate, or vigorous) and the type of exercise (aerobic vs. resistance, or both).17
Approaches to improve physical well-being may include:
- Advice in relation to weight management and avoidance of sedentary behaviour17, 18 (see Chapter 6, Lifestyle Issues).
- Physical activity recommendations should be made in line with those proposed by the National Kidney Foundation (NKF) and Kidney Disease: Improving Global Outcomes (KDIGO).17, 19
- Pre-habilitation to improve frailty and cardiopulmonary fitness with added benefits for mental wellbeing.20
- Referral to physiotherapy services may be indicated to promote the adoption of exercise and raise awareness of the importance of physical activity.19
10.3.1.3 Smoking cessation
We recommend smoking cessation, with referral to local support services if necessary. Patients should also be advised to desist from the use of recreational drugs and moderate alcohol intake.17
10.3.1.4 Diet
Referral to a renal dietitian is recommended to provide personalised advice (see Section 6, Lifestyle Issues).
10.4 Re-transplantation
10.4.1 Patient identification
All transplant patients should be considered for re-transplantation if there is no absolute contraindication (see section 10.4.2). Patients considered for re-transplantation should be discussed in an MDT environment with decisions documented then shared with patients and their core support group. Although there is the potential for selection bias, large cohort and population-level studies have demonstrated greater patient survival for those receiving a second kidney allograft compared to those remaining on dialysis21, 22 (see Chapter 11, Outcomes). Patient and medical decisions about re-transplantation are likely to be more complex as patients are older and likely to be more comorbid compared to their first transplant.23
10.4.2 Suitability for re-transplantation
Absolute and relative contraindications to re-transplantation include:
- Active malignant disease or undergoing post-cancer wait time
- Severe extrarenal disease
- Uncontrolled autoimmune disease
- Ongoing uncontrolled infection
- Cardiovascular morbidity
- Anatomical limitations (e.g. frozen pelvis or occluded vessels)
- Rapidly recurrent disease
- Frailty or cognitive decline
- Weight extremes (high or low BMI)
- Psychiatric or psychological illness
- Poor concordance
Not all contraindications are immutable so re-transplantation should be discussed with the patient (and their core support group) should their situation change and at regular intervals whilst undergoing work-up for RRT or whilst on dialysis.
Patients who are declined transplant re-listing should, if they wish, be offered an opportunity to discuss the decision or have a second opinion from another unit.
10.4.3 Timing
All patients with an eGFR<20ml/minute/1.73m2 or with a graft that is likely to fail within the next 12 months should be considered for re-transplantation. On average renal transplant recipients lose graft function at a rate of 0.8ml/minute/1.73m2/year but this rate of decline is often highly variable and unpredictable.18 This rate of decline may be higher in patients who are younger, female or from Black, Asian and Minority Backgrounds (BAME).24, 25
A timely referral is vitally important as patients with failing transplants are likely to be more comorbid than those undergoing assessment for their first transplant. An automated screening system may help with patient identification. Referral to other specialists and completion of pre-transplant assessments can delay confirmation of fitness to proceed. Due to sensitization, the waiting time on the transplant list may be longer due to the potentially increased complexity of identifying a compatible allograft.26, 27
10.4.4 Living donation
The inaccurate prediction of the timing of transplant failure with late referral is the most frequently identified cause for failing to achieve pre-emptive retransplantation. Timely referral and earlier identification of potential living donors could help to address this issue.28 Several approaches have been developed to encourage living donation, including patient education sessions, living donor champion parties, social media apps involving their core support group, acquaintances or interested parties.29
10.5 Dialysis or conservative management
Patients with a failing kidney transplant should be supported to make informed decisions about their treatment options. The “transplant first” approach may have resulted in a limited knowledge or experience of other RRT modalities or there may have been practice changes since their prior experiences. Thus, treatment options should usually be discussed as though it was their first experience with end-stage renal disease. It is important that all patients are provided with sufficient information and support they need to make use of that information, to enable active participation in the decision-making around their future care.21
Patients and their core support group should be given the opportunity to revisit their decision-making at any stage. We recognise that balance is needed between preserving patient choice and undermining it through continual revisiting decisions.30
10.5.1 Choice of dialysis modality
There is no compelling data to suggest the superiority of either dialysis modality in patients returning to dialysis after allograft failure.31 There are similar outcomes reported for patients starting peritoneal dialysis after the failure of their transplant compared to haemodialysis.32 The choice of dialysis modality should be based on clinical characteristics and patient preference as for patients approaching ESKD for the first time. Most patients become established on haemodialysis.33
Kidney Research UK has published a dialysis decision aid booklet which aims to support patients in deciding which dialysis treatment may fit their individual needs.34 Although this document does not provide specific information for patients who have received a kidney transplant, it may be useful to support their decision-making.
10.5.2 Formation of dialysis access
The unpredictable decline in transplant kidney function makes the timing of dialysis access placement more challenging than for patients with progressive CKD.6 Patients with failing allografts are less likely to undergo dialysis planning leading to inpatient dialysis initiation with associated increased mortality risk.15, 35
Prior arteriovenous access is unlikely to have remained patent. Formation of haemodialysis access should comply with the UKKA guidance, “Vascular access for haemodialysis”, which recommends the formation of an arteriovenous fistula between three and 12 months prior to the expected initiation date. This does not extend to prosthetic grafts which do not require prolonged maturation and can be delayed until closer to the expected date of initiation.36 There is no specific evidence for the timing of peritoneal catheter insertion in patients with failing kidney allografts. We suggest following ISPD and UKKA guidance that catheter implantation should be performed at least two weeks before elective PD initiation.37
10.5.3 Initiation of dialysis
Studies on the optimal timing of dialysis re-initiation following a failed transplant are limited and often conflicting.38We suggest that the timing of dialysis initiation should be based on clinical factors and symptoms related to CKD progression rather than eGFR alone.39
10.5.4 Conservative management
Patients with failing kidney transplants should be offered the choice of conservative management in a similar manner to transplant-naïve patients. It is important to ensure decisions for conservative management between the patient and healthcare team consider the predicted quality of life, predicted life expectancy, comorbidities and care preferences.
In patients undergoing conservative management, it is important to consider balancing the preservation of residual renal function, side-effects and pill-burden of immunosuppressive therapy. In this situation, it is appropriate to adjust immunosuppression to minimise any side-effects (see Chapter 2, Management of Immunosuppression).
10.5.5 Referral to Palliative Care Services
If a patient has chosen conservative management, the need for supportive and palliative care input should be assessed. This may include a formal Psychosocial Needs Assessment as outlined in Section 4 to determine the presence of distress, anxiety or depression as confounders influencing an individual’s reluctance to pursue active treatment/RRT. Some patients may benefit from referral to psychosocial practitioners prior to or in conjunction with palliative care referral.11 When considering referral to palliative care it is important to explain the role of palliative care services to patients and their core support group.
The Gold Standards Framework may be used to (1) identify people needing special care and referral to palliative care services; (2) assess and record their needs; (3) plan and provide care accordingly.40-42
References
- Farrington K, Warwick G. Renal Association Clinical Practice Guideline on planning, initiating and withdrawal of renal replacement therapy. Nephron Clin Pract. 2011;118 Suppl 1:c189-208.
- Dudley C, Harden P. Renal Association Clinical Practice Guideline on the assessment of the potential kidney transplant recipient. Nephron Clin Pract. 2011;118 Suppl 1:c209-24.
- Kochar GS, Langone AJ. How Should We Manage Renal Transplant Patients with Failed Allografts Who Return to Dialysis? Blood Purif. 2020;49(1-2):228-31.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease: assessment and management [NG203]. 2021.
- Stacey D, Legare F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):CD001431.
- Sleiman J, Garrigue V, Vetromile F, Mourad G. Return to dialysis after renal allograft loss: is dialysis treatment initiated too late? Transplant Proc. 2007;39(8):2597-8.
- National Institute for Health and Care Excellence (NICE). Shared decision making [NG197]. 2021.
- Butler CR. A Critical Role for Shared Decision-Making about Referral and Evaluation for Kidney Transplant. Kidney360. 2022;3(1):14-6.
- Amir N, McCarthy HJ, Tong A. A working partnership: A review of shared decision-making in nephrology. Nephrology (Carlton). 2021;26(11):851-7.
- Ucar AR, Demir E, Sever MS. Transplant Patients With Failing Renal Allografts. Exp Clin Transplant. 2018;16 Suppl 1(Suppl 1):4-8.
- Rainer JP, Thompson CH, Lambros H. Psychological and psychosocial aspects of the solid organ transplant experience–a practice review. Psychotherapy (Chic). 2010;47(3):403-12.
- Oncology Nursing Society. Instructional Manual – Instructions for Patient Health Questionnaire (PHQ) and GAD-7 Measures Unknown [Available from: https://www.ons.org/sites/default/files/PHQandGAD7_InstructionManual.pdf.
- Garrubba M. Use of Distress Thermometers in settings outside of oncology 2019 [Available from: https://monashhealth.org/wp-content/uploads/2020/03/Distress-Thermometer_use-in-non-oncology-setting_evidence-snapshot.pdf.
- Marks I. Behavioural psychotherapy in general psychiatry. Helping patients to help themselves. Br J Psychiatry. 1987;150:593-7.
- Chan MR, Oza-Gajera B, Chapla K, Djamali AX, Muth BL, Turk J, et al. Initial vascular access type in patients with a failed renal transplant. Clin J Am Soc Nephrol. 2014;9(7):1225-31.
- Ponticelli C, Favi E. Physical Inactivity: A Modifiable Risk Factor for Morbidity and Mortality in Kidney Transplantation. J Pers Med. 2021;11(9).
- Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1-S115.
- Gill JS, Tonelli M, Mix CH, Pereira BJ. The change in allograft function among long-term kidney transplant recipients. J Am Soc Nephrol. 2003;14(6):1636-42.
- Mallamaci F, Pisano A, Tripepi G. Physical activity in chronic kidney disease and the EXerCise Introduction To Enhance trial. Nephrol Dial Transplant. 2020;35(Suppl 2):ii18-ii22.
- Knectle SJ M, L. P., & Morris, P.,. Kidney Transplantation – Principles and Practice. . 8th ed2019.
- Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 1998;66(12):1651-9.
- Clark S, Kadatz M, Gill J, Gill JS. Access to Kidney Transplantation after a Failed First Kidney Transplant and Associations with Patient and Allograft Survival: An Analysis of National Data to Inform Allocation Policy. Clin J Am Soc Nephrol. 2019;14(8):1228-37.
- Davis S, Mohan S. Managing Patients with Failing Kidney Allograft: Many Questions Remain. Clin J Am Soc Nephrol. 2022;17(3):444-51.
- Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62(1):311-8.
- Hamilton AJ, Plumb LA, Casula A, Sinha MD. Associations with kidney transplant survival and eGFR decline in children and young adults in the United Kingdom: a retrospective cohort study. BMC Nephrol. 2020;21(1):492.
- Lubetzky M, Tantisattamo E, Molnar MZ, Lentine KL, Basu A, Parsons RF, et al. The failing kidney allograft: A review and recommendations for the care and management of a complex group of patients. Am J Transplant. 2021;21(9):2937-49.
- Leal R, Pardinhas C, Martinho A, Sa HO, Figueiredo A, Alves R. Strategies to Overcome HLA Sensitization and Improve Access to Retransplantation after Kidney Graft Loss. J Clin Med. 2022;11(19).
- Alsharani M, Basonbul F, Yohanna S. Low Rates of Preemptive Kidney Transplantation: A Root Cause Analysis to Identify Opportunities for Improvement. J Clin Med Res. 2021;13(1):1-8.
- Hunt HF, Rodrigue JR, Dew MA, Schaffer RL, Henderson ML, Bloom R, et al. Strategies for Increasing Knowledge, Communication, and Access to Living Donor Transplantation: an Evidence Review to Inform Patient Education. Curr Transplant Rep. 2018;5(1):27-44.
- Wong SPY, McFarland LV, Liu CF, Laundry RJ, Hebert PL, O’Hare AM. Care Practices for Patients With Advanced Kidney Disease Who Forgo Maintenance Dialysis. JAMA Intern Med. 2019;179(3):305-
- Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol. 2011;22(6):1113-
- Meng X, Wu W, Xu S, Cheng Z. Comparison of outcomes of peritoneal dialysis between patients after failed kidney transplant and transplant-naive patients: a meta-analysis of observational studies. Ren Fail. 2021;43(1):698-708.
- Laham G, Pujol GS, Vilches A, Cusumano A, Diaz C. Nonprogrammed Vascular Access Is Associated With Greater Mortality in Patients Who Return to Hemodialysis With a Failing Renal Graft. Transplantation. 2017;101(10):2606-11.
- Kidney Research UK. Dialysis: making the right choices for you 2020 [Available from: https://kidneyresearchuk.org/wp-content/uploads/2019/05/KR-decision-Aid-DOWNLOAD.pdf.
- Naylor KL, Knoll GA, McArthur E, Garg AX, Lam NN, Field B, et al. Outcomes of an Inpatient Dialysis Start in Patients With Kidney Graft Failure: A Population-Based Multicentre Cohort Study. Can J Kidney Health Dis. 2021;8:2054358120985376.
- Kumwenda M, Mitra, S., & Reid, C. Clinical practice guideline – Vascular access for haemodialysis 2015 [Available from: https://ukkidney.org/sites/renal.org/files/vascular-access.pdf.
- Crabtree JH, Shrestha BM, Chow KM, Figueiredo AE, Povlsen JV, Wilkie M, et al. Creating and Maintaining Optimal Peritoneal Dialysis Access in the Adult Patient: 2019 Update. Perit Dial Int. 2019;39(5):414-36.
- Messa P, Ponticelli C, Berardinelli L. Coming back to dialysis after kidney transplant failure. Nephrol Dial Transplant. 2008;23(9):2738-42.
- National Institute for Health and Care Excellence (NICE). Renal replacement therapy and conservative management [NG107]. 2018.
- Framework GS. The GSF Centre in End of Life Care 2022 [Available from: https://www.goldstandardsframework.org.uk
- NHS England. End of Life Care in Advanced Kidney Disease: A Framework for Implementation 2017
- Wentlandt K, Weiss A, O’Connor E, Kaya E. Palliative and end of life care in solid organ transplantation. Am J Transplant. 2017;17(12):3008-19.
11. OUTCOMES FOLLOWING RETURN TO DIALYSIS OR RETRANSPLANTATION
Statements of Recommendation
We recommend that:
- Following graft failure, repeat transplantation offers the best patient survival and quality of life. (1C)
- The optimum kidney for retransplantation comes from a pre-emptive well-matched living donor. (1C)
We suggest that:
- Recipients with progressive decline in graft function are worked up in a timely fashion for repeat transplantation if suitable. (1D)
- There is a timely provision of definitive access. (2B)
Recommendations for Audit and Research:
- Clarification of the definition of a failing graft.
- What is the mortality rate of RFKT in the UK?
- How many recipients are there in the UK with failed transplants and what is their current status?
- What is the optimal timing of relisting or pre-emptive transplantation in RFKTs?
- What is contemporaneous survival post graft failure in the UK for those who are relisted or not relisted and remain on dialysis?
- What are the causes of repeated graft loss at a young age and what factors could be mitigated?
Rationale
11.1 Introduction
In this chapter, unreferenced statistics relating to UK transplant outcomes are presented which may be either unpublished or in press. We gratefully acknowledge the support of the Department of Statistics and Clinical Studies, NHS Blood and Transplant (NHSBT). Graft failure is defined as return to renal replacement therapy (peritoneal or haemodialysis or retransplantation) as reported to NHSBT.
11.2 Prevalence of graft failure
Due to the progressive increase in kidney transplantation and improved patient survival, there has been a corresponding increase in recipients with failing kidney transplants (RFKT). In the UK 16% of the 38,895 prevalent transplant recipients on 31/12/2020 had a GFR <30 ml/minute/1.73m2 indicating a poorly functioning or failing renal allograft (24th Renal Registry Report).1 This is in line with 2018 US Renal Data System (USRDS) report where RFKT represent ~4-5% of the incident dialysis population.2
Table 11.1 shows the number of kidney-only graft failures that have been reported to NHSBT between January 2016 and December 2021. This includes kidney-only transplants taking place in the UK from any time point but excludes cases where the recipient died with a functioning graft (DWFG). Thus, this represents graft failure due to any other cause other than death. Prior to 2020, this had been a relatively consistent number. The reduction in 2020 may be due to under-reporting because of the COVID-19 pandemic. This highlights the significant number of patients with a failed graft requiring management of their return to renal replacement therapy or conservative care.
Table 11.1 Annual number of graft failures by donor type excluding DWFG
| Year of graft failure | Number of graft failures | ||
| Deceased donor transplant | Living donor transplant | Total | |
| 2016
2017 2018 2019 2020 |
685
666 711 672 492 |
197
228 232 247 201 |
882
894 943 919 693 |
The median age of recipients at the time of graft failure is similar, regardless of whether this was a first or re-graft, or whether the donor was deceased or living (Table 11.2). This is due to the number of recipients that have repeated transplants at a young age. The table includes grafts with a date of failure between January 2016 and December 2020, but the transplant may have occurred at any time prior. In this cohort, the median age for each subsequent transplant is similar to those experiencing first graft failure. This likely represents skew due to the greater number of transplants in paediatric recipients, where non-adherence is more likely to be a key factor in graft loss. In addition, only the youngest patients will have sufficient life years to be able to have repeated transplants (>2).
Table 11.2 Median age at the time of transplant and subsequent graft failure, failed grafts 1/1/16 – 31/12/20
| Graft number | Deceased Donor | Living Donor | ||||||
| N | Median age at transplant (years) | Median age at graft failure (years) | IQR | N | Median age at transplant (years) | Median age at graft failure (years) | IQR | |
| 1 | 2837 | 46 | 56 | 45-66 | 923 | 37 | 48 | 35 – 59 |
| 2 | 452 | 40 | 52 | 41.5-59 | 153 | 37 | 45 | 36 – 54 |
| 3 | 87 | 40 | 47 | 40-55 | 21 | 41.5 | 46.5 | 40 – 52 |
| 4 | 17 | 48 | 53 | 45-59 | ||||
11.3 Prognosis after graft failure
Graft failure has an adverse impact on patient survival. The risk of death in those with a failed kidney transplant is more than three times higher compared to patients whose transplant continues to function.3 It is well recognised that there is high mortality and morbidity for RFKT returning to dialysis. The meta-analysis by Kabani et al including 250,000 RFKT demonstrates a 12% mortality in the first year post dialysis initiation.4 Although this was limited by study heterogeneity, this value is greater than a comparably aged but unselected international group of haemodialysis patients including high risk unplanned starters5 and those with ESKD on the UK waiting list. However, more recent data demonstrates improvement. A retrospective study by Varas et al of 5216 patients starting dialysis between 2009-2014 found no difference in survival rates between RFKT returning to dialysis and the incident transplant naïve patients when multivariate analysis adjusted for age, sex and comorbidities.6
11.3.1 Death
From those dying post-transplantation, there is a significant number of those who have been reported as a failed graft to NHSBT as shown in table 11.3. Further work would determine whether these patients might have been appropriately worked up for another transplant.
Table 11.3 Patients with failed transplant at the time of death, 1/1/16 – 31/12/20
| Deceased Donor | Living Donor | Total | |
| Number of transplanted patients with functioning graft who died | 3683 | 973 | 4656 |
| Number of deceased patients with a failed graft at the time of death (%) | 877
(23.8%) |
212
(21.8%) |
1089 |
The causes of death post-transplant are shown below in Table 11.4 compared to the prevalent dialysis population. Whilst there are similar proportions of cardiovascular disease and infection, it demonstrates the overrepresented burden of malignancy in causing death post-transplant compared to the prevalent dialysis population.1
Table 11.4 from UK Renal Registry Annual Report shows the cause of death in adult patients prevalent to RRT on 31/12/2018 followed-up in 2019 by modality
11.3.2 Sensitisation
Whilst infection and malignancy are significant causes of co-morbidity in those with failing transplants and might prompt a move to reduce the immunosuppression burden, this must always be balanced with the consequence of sensitisation and alloimmune graft loss.
It is well known that kidney transplantation is a major sensitising event although there is a high variability in the frequency of anti-HLA antibodies detected after transplantation ranging between 1.6-60%.7
The proportion of patients who are highly sensitised (cRF>=85%) on the active transplant list on 31st August 2021 is shown in Table 11.5. There is a similar proportion of highly sensitised patients in those who are suspended.
Table 11.5 – Calculated Reaction Frequency (cRF) of active patients on the transplant list on 31st August 2021
| cRF | N | (%) |
| 0-84% | 3546 | (80%) |
| 85-100% | 904 | (20%) |
Of those highly sensitised, the proportion by graft number is shown in Table 11.6.1. This demonstrates that 67% of those with a cRF =/> 85% are those who have received one or more previous kidney transplant/s. Conversely, of those who are not highly sensitised, less than 10% have had one or more previous transplants. These patients will most likely have avoided sensitisation by receiving a well-matched transplant.
Table 11.6.1 Number of previous transplants in those patients actively listed with cRF=/> 85% and cRF 0 – 85% on 31st August 2021
|
Number of previous transplants |
Number of patients with
cRF =/> 85% (%) |
Number of patients with
cRF 0 – 85% (%) |
|
0 |
298 (33.0%) | 3197 (90.2%) |
|
1 |
469 (51.9%) |
324 (9.1%) |
|
2 |
115 (12.7%) |
24 (0.7%) |
|
3 |
16 (1.8%) |
<5 (<0.1%) |
|
4 |
6 (0.7%) |
– |
The impact of transplantation as a significant sensitising event is demonstrated in the median cRF being at the time of re-transplant (Table 11.6.2).
Table 11.6.2 cRF at transplant, UK kidney only transplants 2016-2020
| Graft Number | N | Median cRF | (Q1-Q3) |
| 1
2 3 4 |
13372
2109 315 48 |
0
76 94 97 |
(0-22)
(25-96) (78-99) (82-99) |
11.3.3 Relisting
In the US, about 85% of RFKTs will never return to the transplant waiting list.8-9 This is similar to the UK. Table 11.7 shows the number of patients with (one or more) failed kidney allografts on the kidney-only transplant list. The low proportion of patients considered for re-transplantation is mainly due to increasing age and comorbidity, and also reflects the high death rate observed after graft failure described above.
Table 11.7 Prevalence of patients with one or more previously failed graft on kidney-only transplant list on 31stAugust 2021
| Total number of patients | Number of patients with previously failed kidney transplant (%) | |
| Active
Suspended |
4450
3502 |
712 (16%)
583 (17%) |
Table 11.8 shows there is a trend towards a lower proportion of patients with a failed kidney transplant being activated on the waiting list. This may represent increasing numbers of ESKD patients being listed for their first allograft, improved graft survival and an increase in pre-emptive living donor re-transplants, as these patients may not have been relisted for deceased donation.
Table 11.8 Prevalence of patients with a previously failed graft in patients joining the active transplant list
|
Year activated |
Number of patients activated | Number of patients activated with a previously failed kidney transplant (%) |
|
2016 |
3502 | 502 (14.3%) |
|
2017 |
3777 | 538 (14.2%) |
|
2018 |
3713 | 499 (13.4%) |
|
2019 |
4080 |
493 (12.1%) |
|
2020 |
2934 |
325 (11.1%) |
It is important to recognise that patients that are suitable to be relisted, wait longer than those without a previous transplant. For patients activated on the deceased donor transplant list between 2010 and 2016, the median time to a deceased donor transplant is shown in Table 11.9. Of note, this does not include those patients who received a living donor transplant, since there is mixed practice in terms of activating patients prior to a living donor transplant. This longer waiting time may in part be accounted for if the previous transplant was from the best-matched living donor and now that is no longer an option.
Table 11.9 Median time (days) to deceased donor transplant for patients spent active on the waiting list 1/1/10 – 31/12/16 from first activation date.
| N | Median | 95% CI | |
| No previous transplant | 14596 | 646 | (634-658) |
| Previous transplant (≧1) | 2572 | 1047 | (998-1096) |
This variation is not evident in the latest USRDS data from 2011 where the median waiting time was 1461 vs 1551 days for first versus subsequent kidney-only transplant.10
Table 11.10 indicates the pre-emptive transplantation rates for DD vs LD for first compared to second transplants. For both DD and LD, fewer second transplants were performed pre-emptively. Several factors may contribute to this. Recipients being considered for re-transplantation may be more medically complex and require a more detailed assessment process, which may not be complete before they start dialysis. Sensitisation may be a consideration, and a second living donor may not be available.
Table 11.10 Proportion of patients on dialysis at the time of transplant, 1/1/16 – 31/12/20
| N on dialysis | (%) | N not on dialysis | (%) | Total | |
| Deceased donor transplant | |||||
| 1st transplant | 8427 | (82.6%) | 1772 | (17%) | 10199 |
| 2nd transplant | 1384 | (90.5%) | 146 | (9.5%) | 1530 |
| Living donor transplant | |||||
| 1st transplant | 2395 | (60.1%) | 1588 | (39.9%) | 3983 |
| 2nd transplant | 459 | (76.6%) | 140 | (23.4%) | 599 |
11.4 Outcome of RFKTs following repeat transplantation
RFKT may choose and be suitable for re-transplantation. From January 2010 to December 2020, 14.6% of deceased donor transplants and 14.2% living donor transplants were repeat kidney only transplants (Table 11.11).
Table 11.11 Number of kidney-only transplants in the UK, 1/1/10 – 31/12/20 by donor type and graft number
| Graft Number | Deceased donor – N (%) | Living donor – N (%) |
| 1
2 3 4 5 |
16899 (85.3)
2454 (12.4) 404 (2.0) 48 (0.2) < 5 |
8960 (85.7)
1254 (12.0) 200 (1.9) 35 (0.3) < 5 |
11.4.1 Patient survival after repeat transplantation
Using data from the Austrian Dialysis and Transplant Registry supplemented by Eurotransplant data, Kainz et alused target trial emulation to compare the difference in restricted mean survival time (RMST) between a second kidney transplant and remaining on the waiting list after a first failed transplant.11 Difference in RMST indicates gain or loss in the event-free survival time due to retransplant or waitlisted but not transplanted during the studied time. Those receiving a second transplant had a longer RMST of 1.6 (95% CI, 0.3 to 2.9) months at 5 years follow-up and 5.8 (95% CI, 0.9 to 11.1) months over 10 years. However, this association was less in patients with longer waiting times since the first graft loss, with no statistically significant survival difference in those waiting more than 3 years.
Data regarding uncensored UK patient survival following a second kidney transplant is shown below. For survival figures in the remainder of this chapter, all antibody-incompatible transplants are excluded from the analysis. Figure 11.2 shows the 5-year patient survival following the 2nd kidney-only transplant, including grafts from January 2010 to December 2016. There does not appear to be any marked perioperative signal of risk in the first few months with a relatively uniform decrease in patient survival following transplantation.
Figure 11.2 Five-year patient survival for patients receiving a 2nd kidney only transplant, 1/1/10 – 31/12/16 by type of transplant.
The survival estimates at 5 years split by donor type are shown in Table 11.12 and the data shows evidence of a difference between donor types (p=0.04, 3df log-rank test). Patients are censored if they received a 3rd transplant.
Table 11.12 – Five-year patient survival estimates following 2nd kidney-only transplant, transplants occurring between 1/1/10 – 31/12/16
| Donor Types | % Patient Survival (%) | 95% CI |
| DD then DD | 90 | (88 – 91) |
| DD then LD | 93 | (90 – 95) |
| LD then DD | 93 | (89 – 95) |
| LD then LD | 95 | (91 – 98) |
Whilst there appears to be a survival advantage from LD followed by LD, this may be influenced by several factors including shorter waiting times due to pre-emptive donation, and donor and recipient age compared to longer time on dialysis for those awaiting DD.
11.4.2 Graft survival after repeat transplantation
UK data demonstrate that those that are suitable for repeat transplantation have good graft outcomes, which are comparable to those captured by the Collaborative Transplant Society that confirms the pan-European outcomes.
Figure 11.3 shows the 5-year graft survival by graft number, for deceased donor kidney-only transplants between January 2010 and December 2016. The survival estimates at 5 years split by graft number are shown in Table 10.15 and the data shows evidence of a difference between graft numbers (p<0.0001, 2df log-rank test). However, there is still a marked survival advantage conferred compared to not transplanting.
Figure 11.3 Five-year graft survival by graft number for deceased donor transplants carried out between 1/1/10 – 31/12/16.
Table 11.13 Five-year graft survival estimates deceased kidney only transplant by graft number, transplants 1/1/10 – 31/12/16
| Graft Number | Five-year Graft Survival (%) | 95% CI |
| 1 | 86 | (86 – 87) |
| 2 | 82 | (80 – 84) |
| 3 | 78 | (72 – 82) |
11.4.3 Effect of donor type on graft survival
The type of donor has an impact on graft survival and this effect is shown below in Figure 11.4 and Table 11.14. Figure 11.4 shows the 5-year graft survival following the 2nd kidney-only transplant, including grafts from January 2010 to December 2016. The number at risk indicates those recipients with a functioning graft for whom there is follow-up at that time point.
Figure 11.4 – Five-year graft survival for patients receiving a 2nd kidney-only transplant, 1/1/10 – 31/12/16.
There is an initial steep drop off in graft survival due to, for example, primary non-function or immune mediated pathology (rapid disease recurrence or hyperacute rejection). Unsurprisingly the best graft survival is seen for LD followed by LD and although it appears that LD then DD has the poorest graft survival, this is not statistically significant compared to DD then DD. The survival estimates at 5 years by donor type are shown in Table 11.14, with evidence of a difference between donor types (p<0.0001, 3df log-rank test).
Table 11.14 Five-year graft survival estimates following 2nd kidney only transplant, transplants occurring between 1/1/10 – 31/12/16
| Donor Types | % Graft Survival | 95% CI |
| DD then DD
DD then LD LD then DD LD then LD |
82
90 80 92 |
(80 – 84)
(86 – 92) (75 – 84) (87 – 95) |
Finally, it is necessary to consider whether any differences in graft survival may be influenced by HLA mismatch. The level of mismatch in living donation is similar regardless of which number graft (Figure 11.5) and whether the previous grafts were LD or DD. Subsequent deceased donor transplants exhibit an increase in level 1 mismatch, similar level 2, decrease in level 3, indicating that recipients are not disadvantaged by a higher mismatch at subsequent transplants.
Figure 11.5 – HLA level by graft number and donor type, UK kidney only transplants 2016-2020.
11.5 Conclusion
RWFT make up a significant number of UK transplant patients and for those that are suitable, a repeat transplant offers graft and patient survival comparable to a first transplant.
References
- https://ukkidney.org/sites/renal.org/files/publication/file-attachments/23rd_UKRR_ANNUAL_REPORT_0.pdf
- United S Renal Data System. USRDS 2011 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015
- Kaplan B, Meier-Kriesche H-U. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2002; 2: 970–974
- Rameez Kabani, Robert R. Quinn, Suetonia Palmer, Adriane M. Lewin, Serdar Yilmaz, Lee A. Tibbles, Diane L. Lorenzetti, Giovanni F.M. Strippoli, Kevin McLaughlin, Pietro Ravani, Alberta Kidney Disease Network, Risk of death following kidney allograft failure: a systematic review and meta-analysis of cohort studies, Nephrology Dialysis Transplantation, Volume 29, Issue 9, September 2014, 1778–1786
- Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis, Kidney Int, 2014, vol. 85 (pg. 158-165)
- Varas J, Perez-Saez MJ, Ramos R et al. Returning to haemodialysis after kidney allograft failure: a survival study with propensity score matching. Nephrol Dial Transplant 2019; 34: 667–672
- Akalin, E. and Pascual, M., 2006. Sensitization after kidney transplantation. Clinical journal of the American Society of Nephrology, 1(3), pp.433-440.
- Rao PS, Ojo A: Organ retransplantation in the United States: trends and implications. Clin Transpl. 2008, 2008: 57-67
- Magee JC, Barr ML, Basadonna GP, Johnson MR, Mahadevan S, McBride MA, Schaubel DE, Leichtman AB: Repeat organ transplantation in the United States, 1996–2005. Am J Transplant. 2007, 7: 1424-1433. 10.1111/j.1600-6143.2007.01786.x.
- https://usrds.org/media/1731/v2_c06_transplant_18_usrds.pdf
- Kainz, A., Kammer, M., Reindl-Schwaighofer, R., Strohmaier, S., Petr, V., Viklicky, O., Abramowicz, D., Naik, M., Mayer, G. and Oberbauer, R., 2022. Waiting time for second kidney transplantation and mortality. Clinical Journal of the American Society of Nephrology, 17(1), pp.90-97