UK Guideline on Management of Bk Polyomavirus (BKPyV) Infection and Disease Following Kidney Transplantation
Table of Contents
Executive Summary of Recommendations
Process of Writing and Methodology
Rationale for Clinical Practice Recommendations for Management of BKPyV Following Renal Transplantation
Executive Summary of Recommendations
1. Laboratory Testing for BKPyV Diagnosis
1.1 Clinical Laboratories should use United Kingdom Accreditation Service (UKAS)-accredited DNA quantitative-Polymerase Chain Reaction (qPCR) assessment of plasma samples to quantify BKPyV viral load. [1C]
1.1.1 BKPyV viral load is reported in many centres as copies/mL or cycle threshold (CT) values in the UK but these are not standardised units of measurement and vary between both assays and sites. BKPyV qPCR assays should be calibrated using the World Health Organization (WHO) International Reference Standard (NIBSC 14/212). [1C]
1.1.2 We should begin work towards reporting in IU/mL, and develop site-specific conversion factors to harmonise nationally collected data and aid interpretation. [2C]
1.1.3 The target for BKPyV DNA qPCR is currently variable between commercial assays with most targeting the VP1 gene and some amplifying other regions of the viral genome. This further hampers comparison of results between sites and stresses the need to use assays calibrated using the WHO International Reference Standard (NIBSC 14/212). [1D]
1.1.4 Transplant centres should adopt a consistent approach to using plasma rather than whole blood for quantification of BKPyV DNAaemia. [1C]
1.1.5 Thresholds in IU/mL on plasma need to be established (this is part of the research recommendations). BKPyV-DNAaemia may be diagnosed as equivocal detection (some centres use >1000 copies/mL) over 2-3 consecutive measurements or one individual confirmed measurement (some centres use >10,000 copies/mL). At present copies/mL thresholds cannot be recommended for national adoption due to the lack of calibration in commonly used assays. Initially, reporting DNAaemia results both in locally-relevant copies/mL and the internationally comparable IU/mL will assist the transition. [1C]
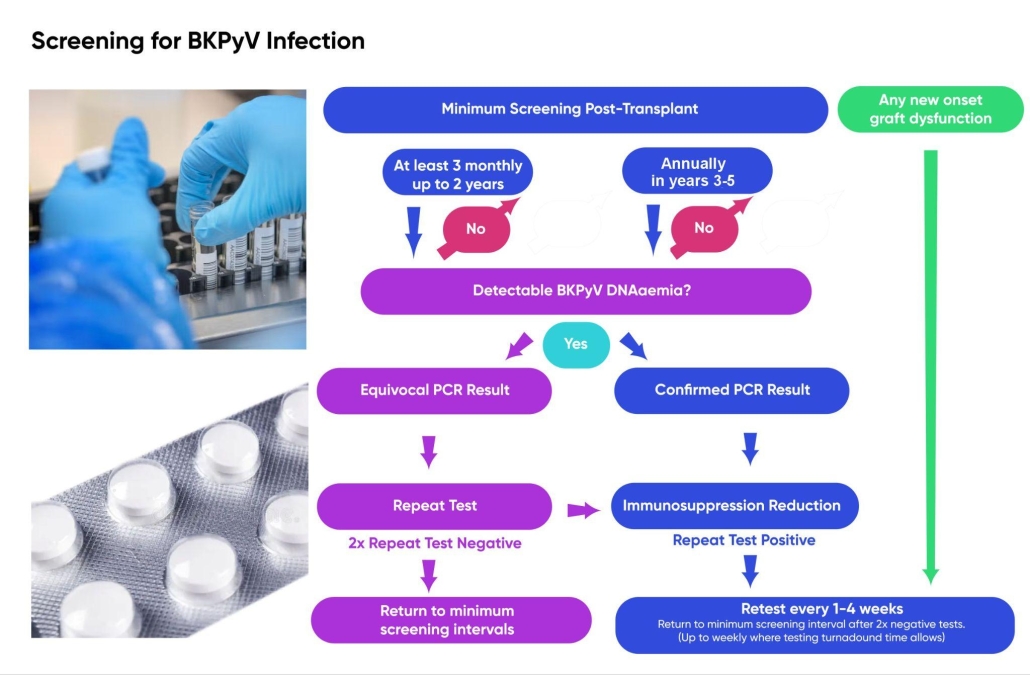
1.2 Whilst randomised controlled trial (RCT) data is lacking in this area and the optimum frequency of testing is unknown, screening is strongly supported by patients. DNA qPCR diagnostic screening intervals of 3 monthly in the first 2 years and annually in years 3-5 post-transplant are recommended as a minimum approach. There may be a case for more frequent (i.e. monthly) monitoring in the first 6 months. We hope that these guidelines will act as a stimulus for further research. [2C]
1.2.1 Frequency of testing needs to incorporate the reality of turnaround times for clinical testing (which nationally varies between 48 hours and 10 days). BKPyV DNA qPCR is generally only available in larger regional virology laboratories. As there are no commercially-available near-patient tests for BKPyV DNA, it is currently not realistic for smaller local laboratories to bring this test in-house. As such, unless the transplant centre is co-located with such a laboratory, samples will be referred to external laboratories, which entails several days’ delay with specimen transport and result transcription, but results should be available within a clinically relevant time period.
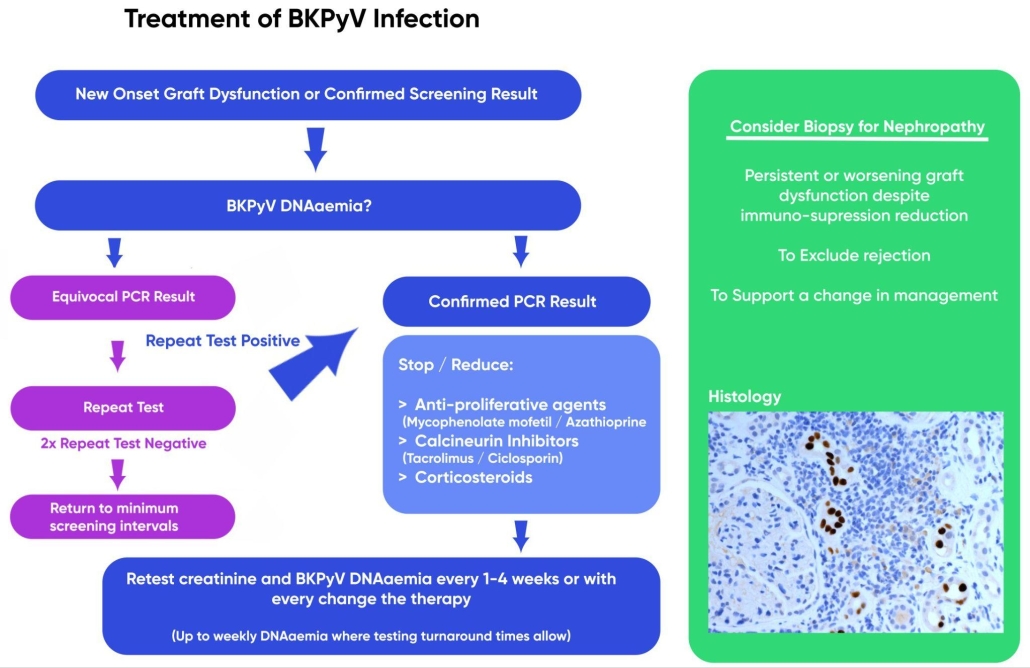
1.3 BKPyV DNA qPCR thresholds should not be used in isolation to determine commencement or cessation of treatment, but rather considered in the context of clinical, laboratory and/or histological evidence of BKPyV disease. [1C]
1.4 BKPyV DNA qPCR on urine is not recommended for monitoring kidney transplant recipients, as positivity is frequent in the ageing general population and not necessarily predictive of BKVAN making interpretation challenging. [1C]
2. Monitoring BKPyV Infection and Disease
BKPyV Associated Nephropathy (BKVAN) is a diagnosis of exclusion. A high index of suspicion is however necessary in the context of a patient who has had significant immunosuppression prior to transplant or a high immunosuppressant burden post transplant. Cases at higher risk of BKVAN include those with high immunological risk, lymphocyte or T-cell depleting therapies or incremented immunosuppression in the context of rejection or incompatible transplants.
Other associations include the occurrence of other viral infections and unexplained lower urinary tract pathologies including ureteric stenosis, haemorrhagic cystitis and bladder cancer.
2.1 Clinical manifestations of acute BKPyV infection:
- BKPyV infections are nearly always asymptomatic [2D]
2.2 BKPyV viral load monitoring is necessary in order to monitor:
- Reactivation of persistent BKPyV infection or de novo acute infections. Frequent testing may be required once infection is established to determine trends in viral load and response to immunosuppression-reduction. [1C]
- Established infection. Minimum frequency of monitoring should be monthly (up to weekly where testing turnaround times allow) until a patient has three consecutive negative BKPyV qPCR tests. A negative test is defined as a result below the limit of detection for plasma specimens using an assay calibrated to the WHO standard. [2C]
- Persistent established infection with low-level DNAaemia (<1,000 copies/mL). Monitoring can be reduced to every 3-6 months providing immunosuppression is not increased. [2C]
- Post-clearance reactivation. DNAaemia screening intervals of 3, 6 and 12 months post-clearance are suggested as a minimum approach (providing immunosuppression is not changed). [2C]
- Resistant infection. Early genotyping may be relevant [1D] and life-long monitoring may be required. [2D]
2.3 For persistent BKPyV DNAaemia, where graft dysfunction is persistent or worsening despite immuno-suppression reduction, consider a graft biopsy. Biopsy can exclude rejection; but should only be performed to support change in management. [2D]
2.3.1 BKVAN is observed in haematoxylin and eosin stained sections by the presence of intranuclear viral inclusions and/or anisokaryosis of the renal proximal tubular epithelial cells.
2.3.2 BKPyV histology (using SV40 Large T Antigen immunolabelling) should be part of the routine testing of all renal biopsies and increases the sensitivity of detection by highlighting infected cells prior to macroscopic cytopathic changes.
3. Immunosuppression Reduction
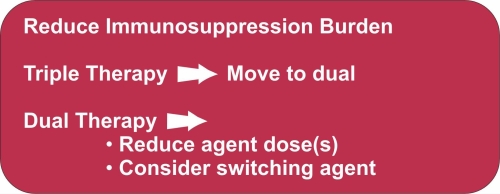
In transplant recipients who develop BKPyV DNAaemia, a clinical decision should be made to cautiously reduce the immunosuppressant burden aiming to avoid precipitating acute or chronic rejection. Careful consideration should be given to the risks of immunosuppression reduction in patients with high immunological risks and stable graft function. Immunosuppression reduction could either be by dose reduction, reducing the number of agents, or substitution of agents.
3.1 Therapy needs to take into consideration initial immunosuppression (at the time of BKPyV detection), viral load, time post-transplant, comorbidities, concurrent rejection, temporal response and expert opinion [1C].
3.2 Discuss with patients, parents or carers the risk of acute rejection with immunosuppression dose reduction [1B]
3.3 Stopping/reducing anti-proliferative agents (mycophenolate mofetil/ azathioprine) or calcineurin inhibitors (tacrolimus/ciclosporin) [1C]
Strategies of approach include:
- Preferentially reducing or stopping anti-proliferative agents or titration of calcineurin inhibitor dose to achieve lower trough concentrations.
- Reducing both anti-proliferative agents and calcineurin inhibitors simultaneously.
3.4 Options for substitution of immunosuppressant agents include:
- Changing from tacrolimus to ciclosporin has limited evidence base and cannot be routinely recommended.
- Introduction of mTOR inhibition as an alternative immunosuppression has been evaluated in several studies; however, there are contradictory findings from small-scale studies and no randomised controlled trials therefore not routinely recommended.
- Substitution of mycophenolate mofetil with Leflunomide has been evaluated in several studies; however, there are contradictory findings from small-scale studies and no randomised controlled trials. This approach cannot be routinely recommended.
3.5 Corticosteroids
- Reduce or stop corticosteroids. [2D]
- For patients not on corticosteroids, the addition of steroids may allow the removal of another component of the immunosuppression regimen. [2D]
- For CNI monotherapy, the addition of steroids may mitigate risk of rejection following CNI trough target concentration reduction. [2D]
3.6 The dose of immunosuppression needs to be reviewed following resolution of BKPyV infection or disease; accounting for a patient’s sensitisation status and presence of donor-specific antibodies. [1D]
4. Treatment of BKPyV Infection
Based on current evidence, the panel cannot recommend any agent as an isolated treatment for managing BKPyV DNAaemia or BKVAN. There are no treatments with robust efficacy data, except for reduction in immunosuppression (discussed in section 3) and none currently have NICE recommendation.
5. Management of Treatment Failure
Treatment failure in the management of BKVAN is defined as persistence of BKPyV graft infection (often monitored using DNAaemia as a proxy) and consequential progressive irreversible graft deterioration leading to kidney transplant graft function loss.
Before diagnosing a patient with BKVAN treatment failure, the following are recommended:
- Perform a kidney transplant biopsy. This ensures clear diagnosis, and the exclusion of other pathology. It also allows prognostication and informs ongoing immunosuppression management [1D]
- Ensure the patient is on the minimised immunosuppression [1D], taking account of the need to balance this against the risk of:
- rejection (both acute and chronic)
- sensitisation (especially for young patients and those needing subsequent grafts)
- Optimisation of treatment through a multidisciplinary approach with transplant physicians, virologists and specialist transplant pharmacists; for instance in monitoring drug concentrations. [1C]
With current clinical practice in the UK, graft loss due to BKPyV infections is an uncommon outcome. Persistent DNAaemia (without evidence of ongoing graft deterioration or histological demonstration of BKVAN) may have implications for prognosis but this has yet to be clinically defined.
Particular attention should be given to paediatric cases of BKVAN where graft loss appears to be more common and paediatric patients are more likely to need re-transplantation.
5.1 In determining the reasons for treatment failure in BKVAN, it is essential to exclude any other cause of transplant failure. Ideally, all graft failures should be evaluated by histology of renal biopsy for the presence of BKPyV, to determine the extent of disease and to distinguish between infection and rejection. [1B]
5.1.1 In cases where a biopsy is SV40 large T antigen positive but the patient was negative for BKPyV DNAaemia, JCPyV DNAaemia testing is clinically appropriate.
5.1.2 Biopsies should be examined for concomitant rejection.
5.2 Previous transplant loss due to BKPyV should not preclude patients from re-transplantation as re-transplantation is often successful following BKVAN in an earlier transplant. Re-transplantation should not be excluded, particularly in the younger patient. [1C]
5.2.1 Ideally, clearance of BKPyV infection / DNAemia should be achieved before re-transplantation. This is often observed when immunosuppression is reduced following graft loss.
5.2.2. Increasing use of ongoing immunosuppression after graft failure (in line with UK and international guidelines) means some patients requiring re-transplantation may not reduce immunosuppression to the level required to see complete clearance of BKPyV DNAaemia. This should not be a contraindication to re-listing.
5.2.3 Some patients may be established on dialysis and still have equivocal BKPyV DNAaemia despite immunosuppression withdrawal. These patients should be monitored for BKPyV DNAaemia every 1-3 months.
5.2.4 Counselling of re-transplanted patients following loss of graft to BKVAN is important to manage the risk of losing the new graft to BKPyV.
5.3 Nephrectomy motivated by clearance of BKPyV infection is controversial and does not have the evidence base required to make recommendations.
6. Information, education and support
6.1 Reliable information should be provided to patients at the point of diagnosis.
6.2 Use the BTS patient information leaflet (PIL), co-developed with patients to give balanced information at initial diagnosis of BKPyV infection in transplant recipients.
6.3 Offer balanced and accurate information about:
- The risks of BKPyV infection and disease
- The nature of immunosuppression changes
- Monitoring approach
6.4 Ensure that healthcare professionals offering information have specialist knowledge about BKPyV infection and disease and their treatment, and the skills to support shared decision-making (for example, presenting information in a form suitable for developmental stage)
Introduction
Biology of BK Polyomavirus in Humans
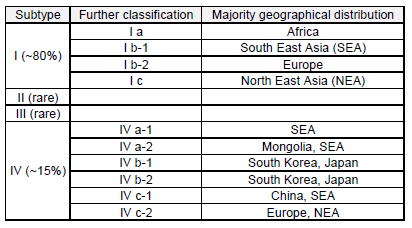
BK polyomavirus (BKPyV) is a member of the Polyomaviridae family of DNA viruses of the genus Betapolyomavirus. It was first isolated in 1971 from a renal transplant recipient with ureteric stenosis who was observed to have abnormal urinary inclusion-bearing cells, later understood to be BKPyV particles [1]. Four major genetic variants of BKPyV have been described and these subtypes can be further divided into subgroups. Subtype I is the most common and has a worldwide prevalence, subtype IV is found in East Asia and Europe and subtypes II and III are rare [2]. Five distinct serotypes, subtype II, III, IV, subgroups Ib1 and 1b2 have been described [2], Table 1.
Table 1: Geographical Distribution of Subtypes [3,4]
The PyV genome
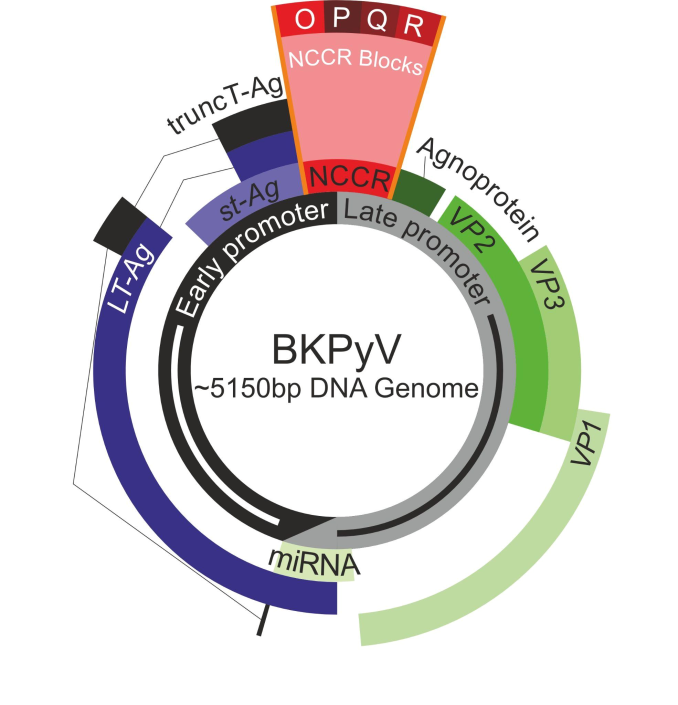
The approximately 5 kilobase circular double-stranded DNA genome of BKPyV is represented schematically in Figure 1.
Expression of the “early” and “late” regions show temporal separation during acute in vitro infections, based on the requirement for Large Tumour Antigen (LT-Ag) protein NCCR binding to drive late gene expression. Clinically observed infections normally display simultaneous expression of all 8 distinct gene products.
Early promoter controlled transcripts:
- LT-Ag
- Small Tumour Antigen (st-Ag)
- Truncated Tumour Antigen (truncT-Ag)
Late promoter controlled transcripts:
- Agnoprotein
- Major Capsid Protein 1 (VP1)
- Minor Capsid Protein 2 (VP2)
- Minor Capsid Protein 3 (VP3)
- Viral microRNA (miRNA)
Figure 1 – Schematic representation of the BKPyV genome. “NCCR” denotes the non-coding control region which is organised into blocks that can be either in an archetypal sequence or a rearranged one.
The “Tumour” Antigens
The BKPyV genome does not encode a DNA polymerase but relies on the viral protein LT-Ag and the infected cell’s DNA repair machinery to replicate the viral genome. LT-Ag is a critical component of the viral life cycle and is the target of some tests to detect BKPyV infections, either by PCR or immunohistochemistry. The main targets for inhibition by LT-Ag are the tumour suppressors p53 and Retinoblastoma protein.
Viral Capsid Proteins (VP1/VP2/VP3)
BKPyV is a small DNA virus with a 40-44 nm (T=7) icosahedral capsid formed of three viral proteins known as VP1, VP2 and VP3 [5]. VP1 is the major component of the viral capsid and the most common target of tests to detect BKPyV infections, either by PCR or immunohistochemistry due to its abundant expression. VP1 plays an important role in viral attachment to cellular receptors [6].
BKPyV infection begins with binding of the major viral capsid protein VP1 to GT1b and GD1B ganglioside receptors and/or α-2 and α-3 sialic acid-containing glycoproteins on the host cell surface [7,8].
Transmission and Seroprevalence
Although maternal antibodies against BKPyV have been observed in babies at birth, these were found to disappear after the first few months of life in a UK observational study [9]. The seroprevalence was found to be 5% in infants aged 4-11 months, and 20-25% by the age of 1 year [9]. There is a rapid increase observed in childhood. The seroprevalence of BKPyV is ubiquitous by adulthood at approximately 95% in some series [10]. There was no observed difference between the distribution in men and women, with no variation between socioeconomic groups and family size [11].
Little is definitively known about the routes of transmission of polyomaviruses. However, BKPyV infection is thought to be acquired in childhood as an asymptomatic or mild respiratory illness, based on a Dutch study that observed BKPyV viral seroconversion amongst 177 children hospitalised for acute respiratory tract infections [12]. Supporting this is evidence of JCPyV in tonsillar tissues [13], MWPyV in adenoid and tonsillar tissues [14] and KI polyomavirus and WU polyomavirus which have also both been found in respiratory secretions [15,16]. Other routes of transmission have been postulated, including sexual transmission and transplacental transmission, based on the detection of BKPyV DNA in sperm and genital tissues [17] and in placental and foetal specimens [18]. Gastrointestinal transmission (following exposure to virus-containing urine/faeces) has also been suggested [19]. BKPyV, JCPyV, KIPyV, WUPyV and MCPyV [20] have all been found in faecal samples of individuals with or without gastrointestinal symptoms. However, given the frequent urinary excretion of BKPyV by the healthy population, a urine-oral route in children cannot be excluded [21].
Persistent Infection
Following an initial acute infection phase, BKPyV establishes a persistent infection of the renal epithelium. Expression of the viral miRNA by archetype BKPyV is abundant and is thought to suppress early promoter activity, dampening the infection and allowing a more quiescent persistent infection to develop [22]. Reactivations of persistent BKPyV infections are more common in renal transplant recipients compared to other solid organ transplant recipients and this direct transmission from kidney donor to recipient provides the main line of evidence for BKPyV persistence in this organ [23,24].
BKPyV Pathophysiology
At this time it is unclear whether BKPyV causes any pathologies in the immunocompetent. However, it is known that BKPyV causes a range of pathologies in the urinary tract of the immunosuppressed including:
- BKPyV Associated Nephropathy (BKVAN)
- Ureteric strictures [25,26]
- Hemorrhagic cystitis (reviewed systematically [27])
- Urothelial cancers of the bladder and ureter. In one study, the BKPyV genome was integrated into 40% of bladder cancers arising in kidney transplant recipients [28]. A meta-analysis suggested a 3.18-fold higher standardised incidence ratio for bladder cancer in patients following renal transplantation [29].
The main complication of BKPyV in kidney transplants is BKVAN, reported in 1-15% of kidney transplants [30]. The first case of biopsy-proven BKVAN was in 1993 and published in 1996 [31]. At that time, graft loss because of BKVAN was 50-100%, but in the last 20 years, this number has reduced to approximately 15% [32].
There have been a small number of reports of BKPyV infection in the brain [33,34] but this requires further investigation.
BKPyV and Kidney Transplantation
BKPyV infections in kidney transplant recipients can originate from three possible sources:
- Reactivation of persistent infection in the host due to immunosuppression
- New infection brought in with the donated kidney
- A new infection arising from an exposure unrelated to the donated organ
The progress of BKPyV infection in renal transplant recipients is associated initially with viruria and decoy cells in the urine, potentially followed by viraemia and then ultimately BKVAN and other pathologies if the infection cannot be controlled (reviewed [35]). Around 30% of renal transplant recipients experience viruria and approximately half of these patients with viruria go on to develop viraemia within 2-6 weeks [30,36–39]. Approximately 12% of renal transplants have detectable BKPyV DNAaemia, with higher DNAaemia being predictive of BKVAN and extensive inflammatory infiltrates [37]. The slow progression of BKPyV infections explain why BKVAN was most commonly observed at 6 months post-transplant [40]. BKVAN is an important cause of renal graft loss.
Early infection is often histologically observed in the distal nephron or medulla and these cells slough/lyse leading to tubulitis and interstitial inflammation as it progresses (reviewed [41]). Later, the proximal nephron and parietal cells of the Bowman′s capsule can also become infected. Generalised tubular injury has also been observed in uninfected tubules [42]. Ultimately cell lysis, loss and inflammation combine to cause tubular atrophy and interstitial fibrosis that reduces the function of the graft to the point that retransplantation may be required.
A critical innate response to infection is the sloughing of infected renal epithelial cells into the urine where they can be detected as “decoy cells” [1]. Cytology for decoy cells is affordable and quick but does not have strong positive predictive value in relation to the development of BKVAN [43–45].
The Need for the Guideline
BKPyV is a leading cause of renal graft function loss and to date there has been no national guideline on the management of this common infection. The lack of guidelines with community engagement and support has led to disparate approaches to screening and management. This lack of unity in approach means care outcomes are variable across Britain and hampers our ability to execute multi-centre clinical trials or establish the standard of best care for this condition.
This first set of BTS Guidelines for the management of BKPyV in renal transplant recipients will not align with the practice in all centres but it is hoped that through engagement with the community they can evolve to define best practice. At this point in time there are no specific anti-BKPyV treatments that can be recommended and this is clearly an element we hope can be revised in future editions of the guidelines as evidence and our approach evolves.
Process of Writing and Methodology
The guideline was developed in accordance with The British Transplantation Society (BTS) Guideline Development Policy [46]. The BTS formed a guideline committee (GC) in November 2022 and a draft scope was developed at a GC meeting in December 2022. Sub-groups of the GC were formed to evaluate the published scientific literature and active clinical trials. Full peer-reviewed papers were then assessed by the GC with a preference for randomised controlled trials (RCT) or non-randomised studies if adjusted for key confounders but accepting that much of the BKPyV literature is currently case study based.
Contributing Authors
Dr Joyce Popoola (Co-Chair), Consultant Nephrologist & Transplant Physician, St George’s University Hospitals NHS Foundation Trust
Dr Simon Baker (Co-Chair), Kidney Research UK Fellow, University of York
Contributors (in alphabetical order):
Dr Mohammed Al-Talib, Cardiff University
Dr Elham Asgari, Consultant in Nephrology and General Medicine, King’s College London and Guy’s and St Thomas’ NHS Foundation Trust
Dr Stephanie Chong, Specialist Registrar in Nephrology, University College London
Dr Rachel Davison, Consultant Nephrologist, South Tyneside and Sunderland NHS Foundation Trust
Mr Daniel Doherty, MRC and JDRF Clinical Research Training Fellow & Higher Surgical Trainee, University of Manchester & Manchester University NHS Foundation Trust
Dr Raymond Fernando, Clinical Scientist at The Anthony Nolan Trust
Dr Rony George, Consultant in Renal Medicine, Northern Care Alliance NHS Foundation Trust
Dr Effrossyni Gkrania-Klotsas, Consultant in Infectious Diseases with an interest in transplantation, Cambridge University Hospitals NHS Trust
Dr Siân Griffin, Consultant Nephrologist in Cardiff and Honorary Senior Lecturer at Cardiff University
Dr Maximillian S. Habibi, Consultant Medical Virologist, St George’s University Hospitals NHS Foundation Trust
Professor Glenville Hargreaves, Patient Representative
Dr Aneesa Jaffer, Renal Academic Clinical Fellow, King’s College Hospital
Dr Pramod Nagaraja, Renal/Medical Specialist Registrar, Cardiff and Vale University Health Board
Dr Nilesh Nanavati, Patient Representative
Dr Ailish Nimmo, Renal Registrar, Royal Infirmary of Edinburgh
Professor Andrew Macdonald, University of Leeds
Professor Stephen D Marks, Professor of Paediatric Nephrology and Transplantation, Great Ormond Street Hospital for Children NHS Foundation Trust and NIHR Great Ormond Street Hospital Biomedical Research Centre, University College London Great Ormond Street Institute of Child Health, London
Mr Omar Masood, Consultant Transplant Surgeon, Leeds Teaching Hospital NHS Trust
Dr Rosa Montero, Nephrologist, St George’s University Hospitals NHS Foundation Trust
Dr Paul Phelan, Consultant Nephrologist & Renal Transplant Physician, Royal Infirmary of Edinburgh
Dr Peter Riley, Consultant Medical Microbiologist, St George’s University Hospitals NHS Foundation Trust
Professor Alan Salama, University College London, Royal Free Hospital
Dr Ahmed Saleh, Renal Registrar, Aberdeen Royal Infirmary
Dr Seema Shrivastava, Consultant Nephrologist, St George’s University Hospitals NHS Foundation Trust
Dr Matthew Welberry Smith, Consultant Nephrologist, MRC Clinical Academic Research Partner on BK virus, Leeds Teaching Hospital NHS Trust
Dr Hannah Wilkinson, Consultant Nephrologist, Imperial College Healthcare NHS Trust
Diagram graphic design by Benjamin Griffiths and histology images courtesy of Dr Rukma Doshi (Histopathologist; St Georges’, Epsom and St Helier Hospitals – GESH).
Conflicts of Interest
None. All authors made declarations of interest in line with the BTS Guideline Development policy. Further details can be obtained on request.
Grading of Recommendations
These guidelines represent consensus opinion from experts in the field of transplantation in the United Kingdom. They represent a snapshot of evidence available at the time of writing. It is recognised that recommendations are made even when the evidence is weak. It is felt that this is helpful to clinicians in daily practice.
In these guidelines the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system has been used to rate the strength of evidence and the strength of recommendations [47]. The approach used in producing the present guidelines is consistent with that adopted by Kidney Disease Improving Global Outcomes (KDIGO) [48,49]. Explicit recommendations are made on the basis of the trade-offs between the benefits on one hand, and the risks, burden, and costs on the other.
The quality of evidence has been graded as:
A (high)
B (moderate)
C (low)
D (very low)
Grade A evidence means high quality evidence that comes from consistent results from well performed randomised controlled trials, or overwhelming evidence of another sort (such as well-executed observational studies with very strong effects).
Grade B evidence means moderate quality evidence from randomised trials that suffer from serious flaws in conduct, consistency, indirectness, imprecise estimates, reporting bias, or some combination of these limitations, or from other study designs with special strength.
Grade C evidence means low quality evidence from observational evidence, or from controlled trials with several very serious limitations.
Grade D evidence is based only on case studies or expert opinion.
A Level 1 recommendation is a strong recommendation to do (or not to do) something where the benefits clearly outweigh the risks (or vice versa) for most, if not all patients.
A Level 2 recommendation is a weaker recommendation, where the risks and benefits are more closely balanced or are more uncertain.
Abbreviations
BKPyV – BK polyomavirus
BKVAN – BK polyomavirus Associated Nephropathy
BTS – British Transplantation Society
DC – Dendritic cell
IVIg – Intravenous Immunoglobulin
NIBSC – The National Institute for Biological Standards and Control
NK cell – Natural Killer cell
mTORi – mammalian target of rapamycin inhibitors
RCT – Randomised Controlled Trial
qPCR – quantitative Polymerase Chain Reaction
UKAS – United Kingdom Accreditation Service
WHO – World Health Organization
Disclaimer
This document provides a guide to best practice, which inevitably evolves over time. All clinicians involved in these aspects of transplantation need to undertake clinical care on an individualised basis and keep up to date with changes in the practice of clinical medicine.
These guidelines represent the collective opinions of a number of experts in the field and do not have the force of law. They contain information/guidance for use by practitioners as a best practice tool. It follows that the guidelines should be interpreted in the spirit rather than the letter of their contents. The opinions presented are subject to change and should not be used in isolation to define the management for any individual patient.
The guidelines are not designed to be prescriptive, nor to define a standard of care. The BTS cannot attest to the accuracy, completeness or currency of the opinions contained herein and do not accept responsibility or liability for any loss or damage caused to any practitioner or any third party as a result of any reliance being placed on the guidelines or as a result of any inaccurate or misleading opinion contained in the guidelines.
References
- Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1: 1253–1257.
- Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Çuburu N, Buck CB. BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J Virol. 2013;87: 10105–10113.
- Morel Virginie, Martin Elodie, François Catherine, Helle François, Faucher Justine, Mourez Thomas, et al. A Simple and Reliable Strategy for BK Virus Subtyping and Subgrouping. J Clin Microbiol. 2017;55: 1177–1185.
- Nishimoto Y, Zheng H-Y, Zhong S, Ikegaya H, Chen Q, Sugimoto C, et al. An Asian origin for subtype IV BK virus based on phylogenetic analysis. J Mol Evol. 2007;65: 103–111.
- Hurdiss DL, Frank M, Snowden JS, Macdonald A, Ranson NA. The Structure of an Infectious Human Polyomavirus and Its Interactions with Cellular Receptors. Structure. 2018;26: 839–847.e3.
- Dugan AS, Gasparovic ML, Tsomaia N, Mierke DF, O’Hara BA, Manley K, et al. Identification of amino acid residues in BK virus VP1 that are critical for viability and growth. J Virol. 2007;81: 11798–11808.
- Sinibaldi L, Goldoni P, Pietropaolo V, Longhi C, Orsi N. Involvement of gangliosides in the interaction between BK virus and Vero cells. Arch Virol. 1990;113: 291–296.
- Neu U, Allen S-AA, Blaum BS, Liu Y, Frank M, Palma AS, et al. A structure-guided mutation in the major capsid protein retargets BK polyomavirus. PLoS Pathog. 2013;9: e1003688.
- Gardner SD. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973;1: 77–78.
- Mentzer AJ, Brenner N, Allen N, Littlejohns TJ, Chong AY, Cortes A, et al. Identification of host-pathogen-disease relationships using a scalable multiplex serology platform in UK Biobank. Nat Commun. 2022;13: 1818.
- Flaegstad T, Rönne K, Filipe AR, Traavik T. Prevalence of anti BK virus antibody in Portugal and Norway. Scand J Infect Dis. 1989;21: 145–147.
- Goudsmit J, Wertheim-van Dillen P, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10: 91–99.
- Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72: 9918–9923.
- Papa N, Zanotta N, Knowles A, Orzan E, Comar M. Detection of Malawi polyomavirus sequences in secondary lymphoid tissues from Italian healthy children: a transient site of infection. Virol J. 2016;13: 97.
- Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MAA, et al. Identification of a third human polyomavirus. J Virol. 2007;81: 4130–4136.
- Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3: e64.
- Monini P, Rotola A, de Lellis L, Corallini A, Secchiero P, Albini A, et al. Latent BK virus infection and Kaposi’s sarcoma pathogenesis. Int J Cancer. 1996;66: 717–722.
- Pietropaolo V, Di Taranto C, Degener AM, Jin L, Sinibaldi L, Baiocchini A, et al. Transplacental transmission of human polyomavirus BK. J Med Virol. 1998;56: 372–376.
- Bofill-Mas S, Formiga-Cruz M, Clemente-Casares P, Calafell F, Girones R. Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J Virol. 2001;75: 10290–10299.
- Prezioso C, Ciotti M, Obregon F, Ambroselli D, Rodio DM, Cudillo L, et al. Polyomaviruses shedding in stool of patients with hematological disorders: detection analysis and study of the non-coding control region’s genetic variability. Med Microbiol Immunol. 2019;208: 845–854.
- Zhong S, Zheng H-Y, Suzuki M, Chen Q, Ikegaya H, Aoki N, et al. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J Clin Microbiol. 2007;45: 193–198.
- Zou W, Vue GS, Assetta B, Manza H, Atwood WJ, Imperiale MJ. Control of Archetype BK Polyomavirus MicroRNA Expression. J Virol. 2020;95. doi:10.1128/JVI.01589-20
- Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Schnitzler MA, Major EO, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5: 2213–2221.
- Schmitt C, Raggub L, Linnenweber-Held S, Adams O, Schwarz A, Heim A. Donor origin of BKV replication after kidney transplantation. J Clin Virol. 2014;59: 120–125.
- Rajpoot DK, Gomez A, Tsang W, Shanberg A. Ureteric and urethral stenosis: a complication of BK virus infection in a pediatric renal transplant patient. Pediatr Transplant. 2007;11: 433–435.
- Chang CYM, Gangji A, Chorneyko K, Kapoor A. Urological manifestations of BK polyomavirus in renal transplant recipients. Can J Urol. 2005;12: 2829–2836.
- Aldiwani M, Tharakan T, Al-Hassani A, Gibbons N, Pavlu J, Hrouda D. BK Virus Associated Haemorrhagic Cystitis. A systematic review of current prevention and treatment strategies. Int J Surg. 2019;63: 34–42.
- Starrett GJ, Yu K, Golubeva Y, Lenz P, Piaskowski ML, Petersen D, et al. Evidence for virus-mediated oncogenesis in bladder cancers arising in solid organ transplant recipients. Elife. 2023;12. doi:10.7554/eLife.82690
- Yan L, Chen P, Chen E-Z, Gu A, Jiang Z-Y. Risk of bladder cancer in renal transplant recipients: a meta-analysis. Br J Cancer. 2014;110: 1871–1877.
- Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347: 488–496.
- Pappo O, Demetris AJ, Raikow RB, Randhawa PS. Human polyoma virus infection of renal allografts: histopathologic diagnosis, clinical significance, and literature review. Mod Pathol. 1996;9: 105–109.
- Wadei HM, Rule AD, Lewin M, Mahale AS, Khamash HA, Schwab TR, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant. 2006;6: 1025–1032.
- Elsner C, Dörries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191: 72–80.
- Bakri FG, Bahou YG, Al-Sammarrai FA, Hadidy A, Gharaibeh A, Zaid GK, et al. Fatal encephalitis due to BK virus in a patient with common variable immunodeficiency: a case report. J Clin Virol. 2013;57: 363–369.
- Chong S, Antoni M, Macdonald A, Reeves M, Harber M, Magee CN. BK virus: Current understanding of pathogenicity and clinical disease in transplantation. Rev Med Virol. 2019;29: e2044.
- Höcker B, Schneble L, Murer L, Carraro A, Pape L, Kranz B, et al. Epidemiology of and Risk Factors for BK Polyomavirus Replication and Nephropathy in Pediatric Renal Transplant Recipients: An International CERTAIN Registry Study. Transplantation. 2019;103: 1224–1233.
- Pollara CP, Corbellini S, Chiappini S, Sandrini S, De Tomasi D, Bonfanti C, et al. Quantitative viral load measurement for BKV infection in renal transplant recipients as a predictive tool for BKVAN. New Microbiol. 2011;34: 165–171.
- Schwarz A, Linnenweber-Held S, Heim A, Framke T, Haller H, Schmitt C. Viral Origin, Clinical Course, and Renal Outcomes in Patients With BK Virus Infection After Living-Donor Renal Transplantation. Transplantation. 2016;100: 844–853.
- Babel N, Fendt J, Karaivanov S, Bold G, Arnold S, Sefrin A, et al. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy: analysis of 4128 urine and serum samples. Transplantation. 2009;88: 89–95.
- Dadhania D, Snopkowski C, Ding R, Muthukumar T, Chang C, Aull M, et al. Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation. 2008;86: 521–528.
- Kant S, Dasgupta A, Bagnasco S, Brennan DC. BK Virus Nephropathy in Kidney Transplantation: A State-of-the-Art Review. Viruses. 2022;14. doi:10.3390/v14081616
- Drachenberg CB, Papadimitriou JC, Wali R, Cubitt CL, Ramos E. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am J Transplant. 2003;3: 1383–1392.
- Maia TMC, Silva SFR, Silva SL, Holanda MC, Nascimento JM, Ferreira MVP. Polyomavirus-infected decoy cells in cytocentrifuged urine cytology specimens from renal transplant recipients. Acta Cytol. 2011;55: 445–448.
- Singh HK, Madden V, Shen YJ, Thompson BD, Nickeleit V. Negative-staining electron microscopy of the urine for the detection of polyomavirus infections. Ultrastruct Pathol. 2006;30: 329–338.
- Geramizadeh B, Roozbeh J, Malek-Hosseini S-A, Azarpira N, Ayatollahi M, Salahi H, et al. Urine cytology as a useful screening method for polyoma virus nephropathy in renal transplant patients: a single-center experience. Transplant Proc. 2006;38: 2923–2925.
- BTS Guideline Development Policy 2021. May 2021 [cited 22 Mar 2024]. Available: https://bts.org.uk/wp-content/uploads/2021/05/BTS_Guideline_Development_Policy_2021.pdf
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328: 1490.
- Uhlig K, Macleod A, Craig J, Lau J, Levey AS, Levin A, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;70: 2058–2065.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3: S1–155.
Rationale for Clinical Practice Recommendations for Management of BKPyV Following Renal Transplantation
1. Laboratory Testing for BKPyV Diagnosis
1.1 Clinical Laboratories should use United Kingdom Accreditation Service (UKAS)-accredited DNA quantitative-Polymerase Chain Reaction (qPCR) assessment of plasma samples to quantify BKPyV viral load. [1C]
1.1.1 BKPyV viral load is reported in many centres as copies/mL or cycle threshold (CT) values in the UK but these are not standardised units of measurement and vary between both assays and sites. BKPyV qPCR assays should be calibrated using the World Health Organization (WHO) International Reference Standard (NIBSC 14/212). [1C]
1.1.2 Work should begin towards reporting in IU/mL, and develop site-specific conversion factors to harmonise nationally collected data and aid interpretation. [2C]
1.1.3 The target for BKPyV DNA qPCR is currently variable between commercial assays with most targeting the VP1 gene and some amplifying other regions of the viral genome. This further hampers comparison of results between sites and stresses the need to use assays calibrated using the WHO International Reference Standard (NIBSC 14/212). [1D]
1.1.4 Transplant centres should adopt a consistent approach to using plasma rather than whole blood for quantification of BKPyV DNAaemia. [1C]
1.1.5 Thresholds in IU/mL on plasma need to be established (part of the research recommendations). BKPyV-DNAaemia may be diagnosed as equivocal detection (some centres use >1000 copies/mL) over 2-3 consecutive measurements or one individual confirmed measurement (some centres use >10,000 copies/mL). At present copies/mL thresholds cannot be recommended for national adoption due to the lack of calibration in commonly used assays. Initially, reporting DNAaemia results in both locally-relevant copies/mL and the internationally comparable IU/mL will assist the transition. [1C]
1.2 Whilst RCT data is lacking in this area and the optimum frequency of testing is unknown, screening is strongly supported by patients. DNA qPCR diagnostic screening intervals of 3 monthly in the first 2 years and annually in years 3-5 post-transplant are recommended as a minimum approach. There may be a case for more frequent (i.e. monthly) monitoring in the first 6 months. We hope that these guidelines will act as a stimulus for further research. [2C]
1.2.1 Frequency of testing needs to incorporate the reality of turnaround times for clinical testing (which nationally varies between 48 hours and 10 days). BKPyV DNA qPCR is generally only available in larger regional virology laboratories. As there are no commercially-available near-patient tests for BKPyV DNA, it is not realistic for smaller local laboratories to bring this test in-house. As such, unless the transplant centre is co-located with such a laboratory, samples will be referred to external laboratories, which entails several days’ delay with specimen transport and result transcription, but results should be available within a clinically relevant time period.
1.3 BKPyV DNA qPCR thresholds should not be used in isolation to determine commencement or cessation of treatment, but rather considered in the context of clinical, laboratory and/or histological evidence of BKPyV disease. [1C]
1.4 BKPyV DNA qPCR on urine is not recommended for monitoring kidney transplant recipients, as positivity is frequent in the ageing general population. [1C]
Research recommendations
In kidney transplant recipients:
- Establish the most appropriate BKPyV target gene for qPCR assays and generate DNA standard to harmonise UK testing allowing national data collection and future clinical trials that meet WHO standards.
- What is the IU/mL threshold for BKPyV qPCR DNAaemia that predicts clinically-relevant BKVAN? To establish an agreed policy on thresholds that determine timing of interventions and provide enrollment criteria for future studies.
- What is the most clinically appropriate or effective and cost-effective duration and frequency of BKPyV DNAaemia screening and monitoring?
- A virtual trial of transplant centres to compare those following the screening guidelines, and those without screening, to determine if there are differences in rate of graft loss due to BKPyV.
- Characterisation of clinical viral genotype to understand whether this defines phenotype.
- Screening organ donors and recipients for BKPyV IgG serostatus and genotype prior to, or at the time of transplantation to establish whether it is predictive of future infection.
- Is there any role for screening of BKPyV viruria? In early screening if adopted what is the risk benefit ratio?
Audit Measures:
- Proportion of adults, children and young people receiving solid organ transplants who are offered or monitored for BKPyV DNA and or have detection on biopsy
Rationale
Screening for DNAaemia
Screening for BKPyV DNAaemia identifies ≥90% of patients at risk of BKPyV renal disease before allograft impairment [50–52].
The quantity of DNAaemia might be important for the progression to fibrosis [53]. In a prospective study of 207 consecutive kidney transplant recipients 57 (28%) developed BKPyV DNAaemia with 10 (5%) cases of BKVAN. Transient (<3 months) DNAaemia occurred in 70% of patients, and persistent (≥3 months) DNAaemia in 30%. A high viral load (≥10 000 copies/mL) was detected in 18% and a low viral load (<10 000 copies/mL) in 61%, while the viral load could not be determined in 21%. Moderate-to-severe interstitial fibrosis and tubular atrophy was significantly increased in high [71%; odds ratio (OR) = 12.1; 95% confidence interval (CI) 1.62-90.0; P = 0.015] or persistent DNAaemia (67%; OR = 6.33; 95% CI 1.19-33.7; P = 0.031) with corresponding rise in “interstitial fibrosis + tubular atrophy” scores. Only patients with transient low BKPyV DNAaemia showed similar incidence and progression of interstitial fibrosis and tubular atrophy to the non-BKPyV group. Persistent low BKPyV DNAaemia was uncommon yet the progression of fibrosis was significant. Only recipients with polyomavirus-associated nephropathy experienced inferior graft survival at 5 years.
Incidental shedding of BKPyV in urine among healthy individuals and among patients immediately prior to transplant has been described in numerous reports. In one prospective study of 220 consecutively enrolled adult and paediatric transplant recipients, pre-implantation BKPyV shedding in urine was detected in 16% of cases. Although numbers were small, this was not predictive of subsequent BKPyV viraemia or BKVAN [54]. Likewise, a Japanese study examining age-related incidence of BKPyV shedding in urine of non-immunocompromised individuals identified a gradual increase in incidence exceeding 30% in those aged over 50, and peaking at 44% among those aged 80-89 [21].
Ideal Screening intervals
The ideal screening intervals for monitoring BKPyV reactivation during different types of immunosuppression remain undefined by randomised controlled trials. The highest incidence of BKPyV DNAaemia and viruria is frequently observed in the third month post-transplantation [55].
Practice surveys differ in their findings. Screening in various European countries in 2015 was usually performed at months 1, 2, 3, 6, 9, and 12 post-transplant [56]. In the UK in 2018, all 23 kidney transplant centres were surveyed, with varying screening intervals reported with most screening every three months in the first year [57].
In Australia, a 2020 survey of nephrologists revealed wide variation in screening practices [58]. The frequency of screening varied between monthly (27%) to 3-monthly (18%) in the first year post-transplant, and was usually more intensive in the first 3 months. Approximately 10% of nephrologists stated they do not routinely perform screening; however, once persistent BKPyV DNAaemia was detected, a third would proceed to allograft biopsy, whereas approximately 70% would consider a biopsy only when graft dysfunction occurred [58].
The American Society of Transplantation Infectious Diseases Community of Practice recommended screening among kidney transplant recipients at months 1, 2, 3, 4, 5, 6, 7, 8, 9, and then 3 monthly until month 24 post-transplant [59]. This is based on studies that suggest that 50-80% of BKPyV DNAaemia cases will develop by 6 months post-transplant [50,60], while 5-15% of cases of DNAaemia will develop between 6 and 12 months, 15% between 12 and 24 months and 5% after month 24 [36,60–63]. Almost 10% of paediatric recipients developed new-onset BKPyV DNAaemia more than 24 months after kidney transplant so screening beyond 24 months post-transplant should be considered in younger patients [36]. Subsequently, screening can be reduced to annually until the fifth year (month 60) post-transplant.
Recommendation of nationally standardised post-transplant screening intervals to allow big data collection
Screening for BKPyV aims to detect early DNAaemia to allow pre-emptive reductions in immune suppression before deterioration in graft function occurs. The optimal frequency of screening intervals for BKPyV has not been examined in any randomised control trial, and existing guidelines vary with respect to their recommended timing of screening, if it is recommended at all.
- A consensus meeting of key stakeholders in 2003 (updated in 2024) recommended that renal transplant recipients be screened at least three monthly during the first two years post-transplant then annually until the fifth year to balance screening efficiency against cost [64,65].
- The 2009 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Care of Kidney Transplant Recipients recommended screening patients for BKPyV monthly for 3-6 months followed by 3 monthly until 12 months post-transplant, when there is an unexplained rise in creatinine, or after treatment for acute rejection [49]. These timings were selected based on the observation that 50% of patients who develop BKPyV DNAaemia do so by 3 months post-transplant, and only 5% of cases occur between 2 and 5 years post-transplant [64].
- In 2017, the Renal Association guidelines on post-operative care of the kidney transplant recipient suggest screening only be carried out when there is an unexplained deterioration in kidney function [66].
- The 2019 American Society of Transplantation Infectious Diseases Community of Practice recommended kidney transplant recipients be screened monthly until month 9, and then 3 monthly until 2 years post-transplant [59].
- In the absence of randomised control trial evidence, a cost-effectiveness analysis of BK screening published in 2022 examined the incremental costs and benefits of routine screening versus no screening in kidney transplant recipients using probabilistic Markov models [67]. This suggested monthly screening using real-time PCR tests for 6 months followed by 3 monthly screening until 12 months post-transplant was cost effective and associated with improved patient outcomes, saving 0.294 life-years and 0.232 quality-adjusted life-years compared with no screening respectively [67].
Consideration needs to be made on whether screening is performed at the same intervals for all transplant recipients, or whether this should be adjusted based on an individual’s level of risk. Risk factors for BKPyV DNAaemia have been described in a number of studies (e.g. summarised in [68,69]). There are different risk factors for early (<6 months post-transplant) and late (>6 months post-transplant) BKPyV DNAaemia. Lymphocyte depleting agents, CMV reactivation and acute rejection appear to be associated with increased early post-transplant viraemia, whilst sensitised patients undergoing repeat transplantation are at increased risk of late BKPyV viraemia [60]. Screening frequency may therefore benefit from being tailored according to immunosuppression use or presence of individual patient-level risk factors.
The World Health Organisation (WHO) created the “1st WHO International Standard for BK Virus DNA” (NIBSC code: 14/212) which aimed at standardising BKPyV DNA detection [70]. The standard can be used to calibrate secondary and/or in-house working standards for local BKPyV DNA assays which allows centres to compare results and pool data [70]. This move towards collecting national data in the UK is essential if we are to engage sufficient participants in clinical trials to gain statistical power. Furthermore, collating national data on BKPyV DNAaemia will help us move towards the generation of thresholds for efficacious clinical interventions.
Variation in pathway
BKPyV screening practice in the UK was examined in a 2018 survey of kidney units in the UK, from which responses were received from all 23 adult transplant centres and 7 non-transplant centres [57]. Of the transplant centres, 16 (70%) performed routine BKV screening but there was variation in the timings and frequency that this was performed. In 6 transplant centres (38%), patients were screened for the first 6 months post-transplant (either by a single screening test at 1, 3 or 6 months, or repeated screening for 3 or 6 months), whilst 7 centres (44%) screened patients for the first year at approximately 3 monthly intervals, 2 centres (13%) screened patients for 2 years post-transplant and 1 centre (6%) continued with screening beyond 2 years post-transplant. Only two of the seven non-transplant centres screened for BKPyV, though all received recipients from centres which undertook screening which would have continued beyond the point of repatriation.
Similar variation in practice has been reported from a survey of nephrologists in Australia, where 27% of the 113 nephrologists responding reported they would screen monthly for 12 months post-transplant, 18% 3 monthly for 12 months post-transplant and 10% of nephrologists not screening at all [58]. A 2015 study in the US also demonstrated variation in practice, with the majority of respondents screening monthly for between 3 and 12 months post-transplant using blood qPCR, with some tailoring their screening approach based on patient characteristics [71].
Understanding the role of BKPyV subtypes in disease
Since its isolation in 1970, the originally isolated Gardner strain or derivative Dunlop strain have been used extensively for research and diagnostic purposes [1]. Both viruses are subtype I and represent the major circulating BKPyV subtype. However, reliance on a small number of lab-adapted viruses risks limiting our knowledge of the BKPyV life cycle to a single subtype. Differences in the infection mechanism have been proposed between BKPyV subtypes [2]. Other differences might exist in the life cycle or in response to therapeutic interventions. Culturing BKPyV clinical isolates has proven difficult; however, all efforts should be made to broaden the available repertoire of BKPyV infectious clones to enable research of all of the major virus subtypes.
References
1. Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1: 1253–1257.
2. Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Çuburu N, Buck CB. BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J Virol. 2013;87: 10105–10113.
21. Zhong S, Zheng H-Y, Suzuki M, Chen Q, Ikegaya H, Aoki N, et al. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J Clin Microbiol. 2007;45: 193–198.
36. Höcker B, Schneble L, Murer L, Carraro A, Pape L, Kranz B, et al. Epidemiology of and Risk Factors for BK Polyomavirus Replication and Nephropathy in Pediatric Renal Transplant Recipients: An International CERTAIN Registry Study. Transplantation. 2019;103: 1224–1233.
49. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3: S1–155.
50. Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13: 136–145.
51. Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10: 2615–2623.
52. Petrov R, Elbahloul O, Gallichio MH, Stellrecht K, Conti DJ. Monthly screening for polyoma virus eliminates BK nephropathy and preserves renal function. Surg Infect . 2009;10: 85–90.
53. Reischig T, Kacer M, Hes O, Machova J, Nemcova J, Kormunda S, et al. Viral load and duration of BK polyomavirus viraemia determine renal graft fibrosis progression: histologic evaluation of late protocol biopsies. Nephrol Dial Transplant. 2019;34: 1970–1978.
54. Verghese PS, Schmeling DO, Filtz EA, Matas AJ, Balfour HH Jr. The impact of recipient BKV shedding before transplant on BKV viruria, DNAemia, and nephropathy post-transplant: A prospective study. Pediatr Transplant. 2017;21. doi:10.1111/petr.12942
55. Koukoulaki M, Grispou E, Pistolas D, Balaska K, Apostolou T, Anagnostopoulou M, et al. Prospective monitoring of BK virus replication in renal transplant recipients. Transpl Infect Dis. 2009;11: 1–10.
56. Pape L, Tönshoff B, Hirsch HH. Perception, diagnosis and management of BK polyomavirus replication and disease in paediatric kidney transplant recipients in Europe. Nephrol Dial Transplant. 2015;31: 842–847.
57. Pyart R. BK polyomavirus practice patterns in the UK – results from a 2018 survey of UK renal centres. British Journal of Renal Medicine. 2020;25: 23–27.
58. Wong G, Marsh J, Howell M, Lim WH, Chadban S, Coates T, et al. Screening and Management Practices for Polyoma (BK) Viremia and Nephropathy in Kidney Transplant Recipients From the Lands Down Under: Addressing the Unknowns and Rationale for a Multicenter Clinical Trial. Kidney Int Rep. 2020;5: 1777–1780.
59. Hirsch HH, Randhawa PS, AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33: e13528.
60. Schachtner T, Babel N, Reinke P. Different risk factor profiles distinguish early-onset from late-onset BKV-replication. Transpl Int. 2015;28: 1081–1091.
61. Leboeuf C, Wilk S, Achermann R, Binet I, Golshayan D, Hadaya K, et al. BK Polyomavirus-Specific 9mer CD8 T Cell Responses Correlate With Clearance of BK Viremia in Kidney Transplant Recipients: First Report From the Swiss Transplant Cohort Study. Am J Transplant. 2017;17: 2591–2600.
62. Bischof N, Hirsch HH, Wehmeier C, Amico P, Dickenmann M, Hirt-Minkowski P, et al. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: long-term outcomes. Nephrol Dial Transplant. 2018;34: 1240–1250.
63. Imlay H, Whitaker K, Fisher CE, Limaye AP. Clinical characteristics and outcomes of late-onset BK virus nephropathy in kidney and kidney-pancreas transplant recipients. Transpl Infect Dis. 2018;20: e12928.
64. Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79: 1277–1286.
65. Kotton CN, Kamar N, Wojciechowski D, Eder M, Hopfer H, Randhawa P, et al. The second international consensus guidelines on the management of BK Polyomavirus in kidney transplantation. Transplantation. 2024;108: 1834–1866.
66. Baker RJ, Mark PB, Patel RK, Stevens KK, Palmer N. Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol. 2017;18: 174.
67. Wong G, Myint TM, Lee YJ, Craig JC, Axelrod D, Kiberd B. Economic Evaluation of Screening for Polyomavirus Infection in Kidney Transplant Recipients: A Cost-Utility Analysis. Transplant Direct. 2022;8: e1318.
68. Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30: 209–217.
69. Dall A, Hariharan S. BK virus nephritis after renal transplantation. Clin J Am Soc Nephrol. 2008;3 Suppl 2: S68–75.
70. 1st WHO International Standard for BK Virus DNA (NIBSC code: 14/212). National Institute for Biological Standards and Control; 2021 Aug. Available: https://nibsc.org/documents/ifu/14-212.pdf
71. Hodowanec AC, Simon DM. BK virus screening and management practices among US renal transplant programs: a survey. Transpl Int. 2015;28: 1339–1341.
2. Monitoring BKPyV Infection and Disease
BKVAN is a diagnosis of exclusion. A high index of suspicion is however necessary in the context of a patient who has had significant immunosuppression prior to transplant or a high immunosuppressant burden post-transplant. Cases at higher risk of BKVAN include those with high immunological risk, lymphocyte or T-cell depleting therapies or incremented immunosuppression in the context of rejection or incompatible transplants.
2.1 Clinical manifestation of acute BKPyV infection
The vast majority of BKPyV infections in kidney transplant patients are asymptomatic.
Whilst clinical assessment would usually suggest BKPyV infections are asymptomatic, and no clear association with any symptom has been robustly established, patients themselves do sometimes associate symptoms with their infections including:
- Malaise, Myalgia and low-grade fever
- Anxiety (this is frequently directly related to BKPyV and the clinical advice to reduce immunosuppression)
- Reduced urine output, difficulty passing urine
- Discomfort over transplant site, abdomen, lower back
- Haematuria, dysuria, or urinary frequency (a urine culture for bacteria should always be sent in these circumstances, rather than assuming these symptoms represent BKPyV)
More rarely reported:
- Cough, colds, or difficulty breathing
- Blurred vision or vision changes
Findings on Investigation
- Rising creatinine
- BKPyV DNAaemia
- BKPyV DNA in urine
- Histological intra-renal polyomavirus load levels (percentage of tubules with active viral replication), although can be highly heterogeneous in the kidney
- Lower urinary tract pathologies including ureteric stenosis and rarely haemorrhagic cystitis.
- The risk of urothelial carcinoma of the bladder or ureters is increased in the years following a transplant.
2.2 BKPyV viral load monitoring is necessary in order to monitor:
- Reactivation of latent BKPyV infection or de novo acute infections. Frequent testing may be required once infection is established to determine trends in viral load and response to immunosuppression-reduction. [1C]
- Established infection. Minimum frequency of monitoring should be monthly (up to weekly where testing turnaround times allow) until a patient has three consecutive negative BKPyV qPCR tests. A negative test is defined as a result below the limit of detection for plasma specimens using an assay calibrated to the WHO standard. [2C]
- Persistent established infection with low-level DNAaemia (<1,000 copies/mL). Monitoring can be reduced to every 3 to 6 months providing immunosuppression is not increased. [2C]
- Post-clearance reactivation. DNAaemia screening intervals of 3, 6 and 12 months post-clearance are suggested as a minimum approach (providing immunosuppression is not changed). [2C]
- Resistant infection. Early genotyping may be relevant [1D] and life-long monitoring may be required. [2D]
2.3 For persistent BKPyV DNAaemia, where graft dysfunction is persistent or worsening despite immuno-suppression reduction, consider a graft biopsy. Biopsy can exclude rejection; but should only be performed to support change in management. [2D]
2.3.1 BKVAN is observed in haematoxylin and eosin stained sections by the presence of intranuclear viral inclusions and/or anisokaryosis of the renal proximal tubular epithelial cells.
2.3.2 BKPyV histology (using SV40 Large T Antigen immunolabelling) should be part of the routine testing of all renal biopsies and increases the sensitivity of detection by highlighting infected cells prior to macroscopic cytopathic changes.
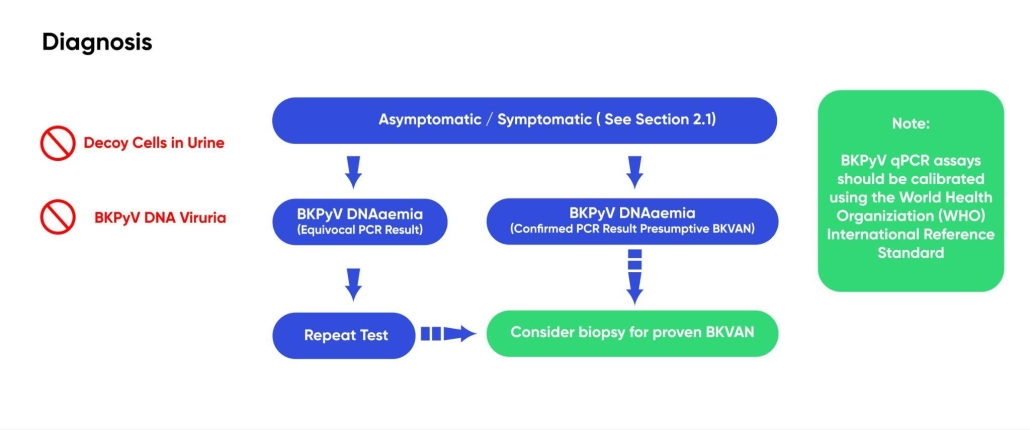
Figure 3 – Flow diagram illustrating the recommended pathway for BKPyV diagnosis. Use of decoy cells in the urine or BKPyV DNA viruria for diagnosis is not recommended.
Research recommendations:
- What is the most clinically- and cost-effective duration and frequency of BKPyV DNAaemia monitoring for the UK population?
- Is there a clinically and cost-effective approach to monitoring of BKPyV immune responses to guide intervention?
- Is there a Gold Standard histological test for diagnosing BKPyV Nephropathy that can be universally adopted?
- Is there a role for assessing the presence and genotype of donor/recipient BKPyV pre-transplant to aid prediction of BKVAN development?
- Can early genotyping in treatment-resistant infection (persistent DNAaemia) identify whether certain BKPyV variants are likely to show poor treatment response?
- What is the best approach to concomitant BKPyV and rejection?
Audit Measures:
- Impact of recipient BKPyV on post-transplant viraemia and/or BKVAN
- Current practice of timing, frequency and duration of BKPyV testing
- Audit to determine the incidence of bladder/upper tract urothelial carcinoma in kidney transplant recipients in association with BKPyV viraemia/nephropathy in the UK
Rationale
Clinical manifestations of BKPyV infection
Finding original source material on symptoms of primary childhood polyomavirus infections has proven impossible; however, an unreferenced paradigm is established in the literature where paediatric infection is either asymptomatic or associated with mild upper respiratory tract symptoms. The association with respiratory symptoms appears mainly based on a single 1982 study showing BKPyV seroconversion amongst 7/177 children hospitalised for acute respiratory tract infections and subsequent detection of BKPyV in the tonsils of these children [12]. Other polyomaviruses are also detected in respiratory tract tissues suggesting this may be a route of transmission [13–16]. This work is relevant to the transplant community because respiratory epithelia may become infected during viraemia and potentially explains patient reports of respiratory symptoms during BKPyV reactivation.
In vitro studies suggest BKPyV will infect almost any cell type [72], such that while direct evidence linking active tissue infections to symptoms is lacking, clinicians should consider the possibility that patient-reported symptoms may be linked to the virus. In particular, BKPyV is known to readily infect the urothelium and its cytopathic effects underpin the haemorrhagic cystitis frequently observed in stem cell transplant recipients [27]. A milder form of bladder pain or cystitis might therefore be an expected complication of BKPyV viraemia/viruria in the renal transplant community.
Transplant recipients may be less likely to perceive nephritis due to variable reinnervation in their grafts and underpins the requirement for effective screening in order to intervene prior to graft failure. BKVAN is a significant cause of graft loss, with one Brazilian observational study of 553 kidney transplant recipients reporting around 6 years of reduced graft survival in patients who developed BKPyV DNAaemia (p<0.001; [73]). Creatinine rises are frequently associated with the early phases of BKVAN but this is not observed in all studies [74–76].
In terms of lower tract symptoms, ureteric strictures are a feature of BKPyV infections where cytopathic damage of the urothelium can lead to occlusion of the ureteric lumen [25,26]. The risk of urothelial carcinoma of the bladder or ureters is increased in the years following a transplant, with a meta-analysis suggesting a 3.18-fold higher standardised incidence ratio [29] and an emerging link to the induction of APOBEC3A/B cytosine deaminases in the tissue [77]. In one study, the BKPyV genome was integrated into 40% of bladder cancers arising in kidney transplant recipients (an event that is very rare in the immunocompetent) [28].
In summary, the absence of quality data linking BKPyV to specific symptoms may be taken as support for the concept that the vast majority of active infections are asymptomatic.
A role for BKPyV Genotype in Pathology?
To date small scale studies have not found any differences in outcomes of infection for the four BKPyV genotypes [78]. However, there is some evidence that BKPyV genotypes are able to escape neutralisation from antibodies raised against another genotype due to variation in receptor binding [2]. This escape may reflect a change in interaction with the sialylated glycans used for viral entry and would therefore suggest altered tropisms between the serotypes and in turn should be hypothesised to influence pathology. There is also recent evidence that host APOBEC-activity might cause VP1 mutations that could alter tropism during infection [79]. The lack of data in this area underpins the recommendation for early genotyping and further research into differences between the genotypes/serotypes.
Role for graft biopsy
A proven diagnosis of BKVAN requires a graft biopsy; however, the clinical circumstances that warrant a biopsy are a matter of debate.
A recent retrospective cohort study correlating BKPyV viral load with evidence of BKVAN on histology suggested that plasma viral load was highly diagnostic (ROC-AUC 0.95) of SV40 positivity on biopsy [80]. SV40 positivity in a biopsy was highly unlikely (ROC-AUC 0.99) where plasma viral load was <4 log10copies/ml [80]. These authors concluded that the distinction between presumptive and histologically proven BKVAN, based on SV40 immunohistochemistry, has limited clinical value [80]. However, the reduction of immunosuppression to manage BKPyV DNAaemia must be considered with both the risk of inducing rejection and exacerbating concomitant rejection.
Clinical teams might consider graft biopsy to establish whether a patient with BKPyV DNAaemia may also have concurrent rejection. A retrospective study of 209 patients who had a graft biopsy at BKPyV diagnosis found that 25 (12%) had evidence of concurrent rejection. Of these, the majority of patients had low level BKPyV DNAaemia (<4 log10 copies/ml). However, a quarter had concurrent BKVAN and rejection [80]. Furthermore, BKPyV is a risk factor for rejection and vice versa, at least in part due to the reduction or augmentation of immunosuppression each necessitates. In one retrospective study, among the 34 patients with high BKPyV viraemia (≥4 log copies/ml), 20% had an episode of graft rejection prior to their BKPyV diagnosis, and 38% developed rejection after their BKPyV diagnosis [81]. Additionally, long-term clinicopathological studies of BKPyV-associated nephropathy assessing biopsies performed after initial diagnosis reported the incidence of acute rejection was 28% in the second biopsy and 50% subsequently [82].
In 2018 the Banff Working Group developed a histological classification system for defining BKVAN [83]. The group found two independent predictors of BKVAN were intrarenal polyomavirus load and Banff interstitial fibrosis ci scores [83]. These two variables were used to define three histological classes of polyomavirus nephropathy that were validated in a cohort of 99 BKVAN patients [83,84]. Despite their rigorous approach, the Banff Classification has not seen widespread adoption either in the UK or further afield. A research application of the Banff classification to 53 patients with BKVAN found that while the three classes did not correlate with BKPyV DNAaemia copies/mL, they did associate with 1-year graft survival [85]. A second application to 124 BKVAN patients found they could not be stratified or identified in terms of allograft failure risk by the Banff Classification [86]. The authors suggest the Banff classification may perform better if class 1 and 2 patients were grouped together, as class three offered some ability to predict graft failure [86]. The original authors of the Banff classification discussed the variance in findings in an opinion piece [87]; however, at present, consensus is still developing on the best histological approach to predicting BKVAN outcomes.
References
2. Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Çuburu N, Buck CB. BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J Virol. 2013;87: 10105–10113.
12. Goudsmit J, Wertheim-van Dillen P, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10: 91–99.
13. Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72: 9918–9923.
14. Papa N, Zanotta N, Knowles A, Orzan E, Comar M. Detection of Malawi polyomavirus sequences in secondary lymphoid tissues from Italian healthy children: a transient site of infection. Virol J. 2016;13: 97.
15. Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MAA, et al. Identification of a third human polyomavirus. J Virol. 2007;81: 4130–4136.
16. Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3: e64.
25. Rajpoot DK, Gomez A, Tsang W, Shanberg A. Ureteric and urethral stenosis: a complication of BK virus infection in a pediatric renal transplant patient. Pediatr Transplant. 2007;11: 433–435.
26. Chang CYM, Gangji A, Chorneyko K, Kapoor A. Urological manifestations of BK polyomavirus in renal transplant recipients. Can J Urol. 2005;12: 2829–2836.
27. Aldiwani M, Tharakan T, Al-Hassani A, Gibbons N, Pavlu J, Hrouda D. BK Virus Associated Haemorrhagic Cystitis. A systematic review of current prevention and treatment strategies. Int J Surg. 2019;63: 34–42.
28. Starrett GJ, Yu K, Golubeva Y, Lenz P, Piaskowski ML, Petersen D, et al. Evidence for virus-mediated oncogenesis in bladder cancers arising in solid organ transplant recipients. Elife. 2023;12. doi:10.7554/eLife.82690
29. Yan L, Chen P, Chen E-Z, Gu A, Jiang Z-Y. Risk of bladder cancer in renal transplant recipients: a meta-analysis. Br J Cancer. 2014;110: 1871–1877.
72. An P, Sáenz Robles MT, Duray AM, Cantalupo PG, Pipas JM. Human polyomavirus BKV infection of endothelial cells results in interferon pathway induction and persistence. PLoS Pathog. 2019;15: e1007505.
73. Moura EB, Petzhold SV, Amaral AR, Deboni LM, França PHCDE. Evaluation of the predisposition and clinical impact of BK virus replication in kidney transplant patients. An Acad Bras Cienc. 2017;89: 675–684.
74. Ramos E, Drachenberg CB, Papadimitriou JC, Hamze O, Fink JC, Klassen DK, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13: 2145–2151.
75. Chen X-T, Li J, Deng R-H, Yang S-C, Chen Y-Y, Chen P-S, et al. The therapeutic effect of switching from tacrolimus to low-dose cyclosporine A in renal transplant recipients with BK virus nephropathy. Biosci Rep. 2019;39. doi:10.1042/BSR20182058
76. Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5: 582–594.
77. Baker SC, Mason AS, Slip RG, Skinner KT, Macdonald A, Masood O, et al. Induction of APOBEC3-mediated genomic damage in urothelium implicates BK polyomavirus (BKPyV) as a hit-and-run driver for bladder cancer. Oncogene. 2022;41: 2139–2151.
78. Toan PQ, Bao Quyen LT, Thu Hang DT, My Anh TT, Cuong LM, Lanh NS, et al. Identification of BK Virus Genotypes in Recipients of Renal Transplant in Vietnam. Transplant Proc. 2019;51: 2683–2688.
79. Sorin MN, Di Maio A, Silva LM, Ebert D, Delannoy CP, Nguyen N-K, et al. Structural and functional analysis of natural capsid variants suggests sialic acid-independent entry of BK polyomavirus. Cell Rep. 2023;42: 112114.
80. Cleenders E, Koshy P, Van Loon E, Lagrou K, Beuselinck K, Andrei G, et al. An observational cohort study of histological screening for BK polyomavirus nephropathy following viral replication in plasma. Kidney Int. 2023;104: 1018–1034.
81. Lee S, Lee KW, Kim SJ, Park JB. Clinical Characteristic and Outcomes of BK Virus Infection in Kidney Transplant Recipients Managed Using a Systematic Surveillance and Treatment Strategy. Transplant Proc. 2020;52: 1749–1756.
82. Drachenberg CB, Papadimitriou JC, Chaudhry MR, Ugarte R, Mavanur M, Thomas B, et al. Histological Evolution of BK Virus-Associated Nephropathy: Importance of Integrating Clinical and Pathological Findings. Am J Transplant. 2017;17: 2078–2091.
83. Nickeleit V, Singh HK, Randhawa P, Drachenberg CB, Bhatnagar R, Bracamonte E, et al. The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: Morphologic Definitions and Clinical Correlations. J Am Soc Nephrol. 2018;29: 680–693.
84. Nickeleit V, Singh HK, Dadhania D, Cornea V, El-Husseini A, Castellanos A, et al. The 2018 Banff Working Group classification of definitive polyomavirus nephropathy: A multicenter validation study in the modern era. Am J Transplant. 2021;21: 669–680.
85. Wang M, Zhou Q, Wang H, Chen Y, Chen J. An application of the 2018 Banff Classification for BK polyomavirus-associated nephropathy in renal transplantation. Transpl Infect Dis. 2021;23: e13557.
86. Kowalewska J, El Moudden I, Perkowska-Ptasinska A, Kapp ME, Fogo AB, Lin MY, et al. Assessment of the Banff Working Group classification of definitive BK polyomavirus nephropathy. Transpl Int. 2021;34: 2286–2296.
87. Nickeleit V, Singh HK, Davis VG, Seshan SV. Classifying Polyomavirus nephropathy: The “Banff” initiative. Transpl Int. 2022;35: 10299.
3. Immunosuppression Reduction
In transplant recipients who develop BKPyV DNAaemia, a clinical decision should be made to cautiously reduce the immunosuppressant burden aiming to avoid precipitating acute or chronic rejection. Careful consideration should be given to the risks of immunosuppression reduction in patients with high immunological risks and stable graft function. Immunosuppression reduction could either be by dose reduction, reducing the number of agents, or substitution of agents.
3.1 Therapy needs to take into consideration initial immunosuppression (at the time of BKPyV detection), viral load, time post-transplant, comorbidities, concurrent rejection, temporal response and expert opinion [1C].
3.2 Discuss with patients, parents or carers the risk of acute rejection with immunosuppression dose reduction [1B]
3.3 Stopping/reducing anti-proliferative agents (mycophenolate mofetil/ azathioprine) or calcineurin inhibitors (tacrolimus/ciclosporin) [1C]
Strategies of approach include:
- Preferentially reducing or stopping anti-proliferative agents or titration of calcineurin inhibitor dose to achieve lower trough pre-dose concentrations.
- Reducing both anti-proliferative agents and calcineurin inhibitors simultaneously.
3.4 Options for substitution of immunosuppressant agents include:
- Changing from tacrolimus to ciclosporin has limited evidence base and cannot be routinely recommended.
- Introduction of mTOR inhibition as an alternative immunosuppression has been evaluated in several studies; however, there are contradictory findings from small-scale studies and no randomised controlled trials therefore not routinely recommended.
- Substitution of mycophenolate mofetil with Leflunomide has been evaluated in several studies; however, there are contradictory findings from small-scale studies and no randomised controlled trials. This approach cannot be routinely recommended.
3.5 Corticosteroids
- Reduce or stop corticosteroids. [2D]
- For patients not on corticosteroids, the addition of steroids may allow the removal of another component of the immunosuppression regimen. [2D]
- For CNI monotherapy, the addition of steroids may mitigate risk of rejection following CNI trough target concentration reduction. [2D]
3.6 The dose of immunosuppression needs to be reviewed following resolution of BKPyV infection or disease; accounting for a patient’s sensitisation status and presence of donor-specific antibodies. [1D]
Figure 4 – Flow chart illustrating the recommended approach to treatment.
Research recommendations:
- UK-wide multicentre randomised controlled trials are strongly recommended.
- Trial strategy to harmonise immunosuppression including dose reduction strategy.
- In those who develop BKPyV infection or disease following solid organ transplantation; is introduction of tacrolimus/sirolimus or replacement with sirolimus associated with better outcomes?
- Assess the trajectory of response using current regimens including establishing the frequency of monitoring.
Audit Measures:
- Current centre-specific practice of immunosuppression reduction against local guidelines.
- Where variation in local practice for immunosuppression reduction exists, audit effect on BKVAN outcomes.
- Frequency of graft loss in spite of immunosuppression reduction.
Rationale
Immune response to BKPyV infection
Immune responses to BKPyV infection, through both the innate and adaptive immune system are critical for control of viral replication and the prevention of pathogenesis.
The innate immune system provides the first line of defence for viral control. However, these early defence mechanisms are not always robust enough to clear viral infection [88]. Neutrophils perform part of the early response to viral infections but their role in fighting BKPyV remains unclear [89]. Likewise eosinophils may be recruited to the site of infection but their role has attracted limited research [90]. Macrophages are known to be recruited to BKPyV infection sites although the expression of aryl hydrocarbon receptor may indicate these cells take on a less inflammatory M2 phenotype [91].
Natural Killer (NK) cells form part of the innate response (although possessing some adaptive qualities) and recognise virally infected cells, triggering cytotoxicity to control infections. The tempo of NK cell response is dictated in part by signalling through killer-cell immunoglobulin-like receptors (KIRs; reviewed [92]) where lower expression of activating KIR3DS1 has been associated with severe BKVAN in kidney transplant recipients [93]. The BKPyV miRNA can suppress the expression of ULBP3, a stress-induced ligand recognised by the NK receptor “NKG2D”, reducing NK cell-mediated cytotoxicity [94]. Specific HLA loci may have a protective role when present kidney transplant donors and recipients however require further research [23].
An effective immune response requires a precise crosstalk among the various immune cells as an intercellular signalling network. These cytokines and chemokines control the magnitude and tempo of the immune response. Tumour necrosis factor alpha (TNFα) and interferon gamma (IFNγ) have been demonstrated as major cytokines for controlling the spread of the BKPyV in kidney-transplanted recipients with BKVAN [95]. However, TNFα has also been suggested to stimulate BKPyV replication in renal proximal tubular epithelial cells [96]. Both interferons alpha and gamma have been shown to restrict BKPyV replication in renal proximal epithelial cells [97,98] and for gamma this has been reproduced in urothelial cells [77].
Adaptive immunity
Dendritic cells (DCs) play a critical role in clearance of virally infected cells (reviewed [99]) and a reduced number of DCs have been observed in the peripheral blood of kidney transplant recipients with BKVAN [100]. It is likely that the reduction in DC leads to a weaker BKPyV adaptive response given DC are potent antigen-presenting cells important for the induction of antiviral cytotoxic T-cell responses. In mouse models, the magnitude of murine polyomavirus specific CD8+ T cell responses correlated directly with the amount of DC [101,102]. Interestingly, unlike other polyomaviruses, the VP1 protein of BKPyV does not induce DC maturation [103].
IgG seropositivity can reach 95% in the general population [10] and IgG seroreactivity tends to increase in those with active viral replication [104]. The presence of BKPyV-specific antibodies alone does not provide protection against BKPyV reactivation and its associated pathology [104]. The lack of neutralisation despite high antibody titre could be due to mutations in the viral capsid genes; as the main BKPyV genotypes can reciprocally escape neutralisation by antibodies raised against the other genotypes [2]. More studies focussed on anti-BKPyV neutralising antibodies are needed.
The role of the T cell response in clearance of BKPyV infection post kidney transplantation has been extensively reported [105,106]. There is a direct correlation between circulating levels of BKPyV-specific CD8+ T cells and effective immune control [107] suggesting a potential role for monitoring BKPyV-specific T cells in the peripheral blood of kidney transplant recipients to predict BKPyV-reactivation as done for CMV [108]. In the post-transplant period prompt recovery of BKPyV-specific T cells protects against reactivation where an insufficient BKPyV-specific T cell immunity is associated with continued viral replication and inflammation [105].
Both CD4+ and CD8+ T cell responses have a role in control of BKPyV infection [109]. Some reports have suggested that the CD4+ immune response plays a greater role in controlling BKPyV infection than CD8+ T cells [110] as evidenced by the ability of the CD4+ T helper cells to control BKPyV reactivation in the absence of a CD8+ response [95]. This CD4+ mediated control of BKPyV replication is through the expression of IFNγ, TNFα and granzyme B [95]. Polyomavirus miRNA activity has also been shown to have a role in mediating immune evasion by making virally infected cells less susceptible to recognition by cytotoxic T cells and reducing the production of cytokines by these cells [111].
BKPyV-specific T cell responses have been demonstrated against the BKPyV T antigens and capsid proteins in both seropositive healthy individuals and transplant patients [105,112–115]. By contrast, the BKPyV agnoprotein is abundantly expressed but largely ignored immunologically [116]. Data from kidney transplant recipients with active BKPyV replication suggested that CD8+ T cells were predominantly LT-Ag specific, while VP1 elicited a mainly CD4+ T cell response [117]. VP3 has been shown to be an antigenic target eliciting both CD4+ and CD8+ T cell responses [118]. Within the renal tissue itself the presence of BKPyV-specific T cells has been associated with both BKPyV viremia and BKVAN [119]. Critically, in the same study, renal biopsy specimens contained 7.8-times more alloreactive T cell clones than BKPyV LT-Ag-specific T cells [119].
At least 39 distinct CD4+ and CD8+ T cell epitopes for the BKPyV early viral gene region have been identified and there is some homology between BKPyV-specific and JCPyV-specific T cell epitopes [120]. Furthermore there has been reported overlap between BKPyV and JCPyV immune responses where prior JCPyV infection may induce T cell responses which are able cross-protect from BKPyV-associated diseases, and vice versa, due to the homology between polyomaviruses [121–123].
Immunosuppression Management in BKVAN
BKVAN is associated with over-immunosuppression, allowing re-emergence and replication of the dormant BKPyV. There are no randomised controlled trials addressing management approaches. Comparison of available evidence is limited by differing definitions of immunological risk, immunosuppressive regimes and diagnosis of BKVAN.
The first strategy in confirmed BKVAN is to reduce immunosuppression. This can lead to resolution of BKVAN in anywhere between 25% and 95% of cases depending on the definition of ‘cure’ used [51,75,76,124–126]. With increases in the number and proportion of highly sensitised patients on national kidney transplant waiting lists, and the advent of increasing transplantation across antibody barriers, immunosuppression reduction has become a more complex consideration, and may not be appropriate in all clinical situations e.g. where concurrent donor specific antibodies are detectable. This is particularly a problem for the paediatric and young adult population who almost inevitably will require re-transplant as demonstrated by registry data [127].
Some studies have suggested the use of specific immunosuppressive medications are associated with a higher risk of BKVAN and other studies have suggested that it is the overall burden of immunosuppression that is relevant [51,76,125,126,128,129].
Induction Therapies
Bayraktar et al performed a retrospective analysis of 257 adult patients at their single centre in Turkey who underwent transplantation between 2007 and 2017 stratified by induction therapy (none, ATG alone, ATG plus basiliximab) based on immunological risk [128]. No relationship was found between the incidence of BKVAN and the induction agent used (p>0.05). All patients received standard treatment with tacrolimus, MMF and corticosteroids.
Hassig et al reported no relationship between BKVAN and the use of basiliximab compared to ATG induction (p=0.212, [129]) as did Radtke et al [130] and Jacobi et al [131]. This is in contrast to Dadhania et al who reported ATG to be associated with an increased risk of BKVAN in comparison to basiliximab [40]. Saull et al compared the use of alemtuzumab and ATG as induction agents in primary non-sensitised renal transplant patients who underwent rapid steroid withdrawal (by day 5 post-transplant) [132]. All patients received tacrolimus and MMF maintenance therapy [132]. This single centre retrospective analysis of 200 consecutive patients showed that the one year rates of BKPyV DNAaemia (defined as BKPyV DNA copies/ml >5000) were identical between the two groups (p=0.36) but BKVAN (defined as positive immunohistochemistry staining for BKPyV on protocol biopsy) was more frequent in the alemtuzumab arm (p=0.046) [132]. The two UK based single centre trials of alemtuzumab induction in low immunological risk renal transplantation did not show any difference in BKPyV DNAaemia or BKVAN rates [133,134]. Another single centre retrospective review of 666 patients where 80% received alemtuzumab did not find a significant difference in the incidence of BKPyV DNAaemia or nephropathy in those who received depleting agents [135]. It was noted that BKPyV viraemia/BKVAN was observed frequently before an episode of biopsy-proven acute rejection which the authors attributed to a reduction in maintenance immunosuppression to treat BKPyV although they did not specify the regime they used [132].
Maintenance Immunosuppression
There have been multiple case reports and cohort studies looking at reduction of maintenance immunosuppression after diagnosis of BKPyV DNAaemia/BKVAN which is a generally accepted common first step in management of BKPyV [64]. However, there is no randomised controlled trial evidence to support reduction of maintenance immunosuppression. A meta-analysis based on the observational data available was only able to confirm reduction of maintenance immunosuppression as an effective strategy [136]. The efficacy of immunosuppression reduction is believed to be in allowing BKPyV-specific T-cells to control the viral reactivation [117,137].
Some authors [138] advocate a reduction in immunosuppression as soon as BKPyV viruria is confirmed, as this is noted to precede DNAaemia [76,139], and clinically evident prior to BKVAN by some weeks; but we do not recommend this due to the poor positive predictive power of viruria. Elfadawy et al adopted a strategy of only reducing immunosuppression in viraemia with BKPyV levels >10,000 copies/ml as they demonstrated spontaneous viral clearance in 95% of patients with lower levels [125], as did Sood et al [140] and Saad et al [141]. Schaub et al commented that viral clearance was more likely to be prolonged with higher viral loads [51]. Cases of BKPyV DNAaemia and BKVAN have been seen with the use of all six of the most commonly used immunosuppressive medications (steroids, CNIs, AZA/MMF and sirolimus) [76,126,142–144] but as they are often used in combination, it has proven challenging to determine if any single agent is more implicated than others.
Two papers have reported BKVAN occurring in the absence of CNI usage [144,145]. Baek et al noted an increased risk of biopsy-proven acute rejection on multivariate analysis if MMF was entirely discontinued or if CNI levels were reduced by more than 20% from baseline [146] but this is not consistently seen in all studies [76]. The observation of BKVAN occurring after an episode of biopsy-proven acute rejection is also common [143,146–148], presumably as a consequence of intensification of immunosuppression in this context.
Reduce Antiproliferatives First?
There is no consistency as to which agents are minimised or what levels are targeted [142]. Most authors [51,75,76,124,125,129,131,137,142,143,146–154] who follow the consensus recommendations proposed by Hirsch et al [59] have reported minimisation (by at least a 50% dose reduction) or complete cessation of MMF in the first instance with secondary reduction in CNI (calcineurin inhibitor) dose (targeting trough tacrolimus levels of <6 ng/ml and ciclosporin of <150 ng/ml). Gheith et al [149] reported resolution of viruria with this approach and targeted trough tacrolimus levels of 6 ng/mL. However, they did not report if this resolved BKVAN histologically. Abdeltawab et al [151] targeted trough tacrolimus levels of 3-6ng/ml and Brennan et al [76] suggested a similar level of 3-5 ng/ml. Jacobi et al [131] used a lower trough ciclosporin level of 60-80 ng/ml. Brennan et al [76] demonstrated no difference in development of BKPyV viraemia between patients receiving MMF or azathioprine in conjunction with CNI therapy.
Tacrolimus vs ciclosporin?
Ott et al supported a change from tacrolimus to ciclosporin after cessation of MMF but did not specify their target level [150]. A number of registry analyses and studies [50,126,154–156] also reported tacrolimus to be more strongly associated with the development of BKVAN but this is not consistent in all studies [76,130,131,147]. Gard et al observed higher BKPyV viral loads at some, but not all time points, in their study of tacrolimus treated patients in comparison to those on a ciclosporin based regime [147]. They also noted lower overall viral load with an earlier decline in titre in ciclosporin treated patients [147]. Multiple authors have shown that ciclosporin has some in vitro antiviral activity by preventing BKPyV replication but this has not yet been confirmed in vivo [117,137,157]. Hirsch et al have also demonstrated that tacrolimus may actually favour BKPyV replication in human tubular epithelial cells by the action of FK-binding protein 12 [50]. Other authors demonstrated a dose-dependent effect by tacrolimus to inhibit BKPyV-specific T-cells [117,137].
There is a single prospective study of patients with biopsy proven BKVAN receiving triple immunosuppression with tacrolimus, MPA and steroids being changed to ciclosporin [75]. In this study of 24 patients over a 2-year period, target trough ciclosporin levels were 75-125 ng/ml (higher than Jacobi et al who used 60-80 ng/ml [131]) whilst maintaining the same dose of other medications [75]. No control group was included, so no direct comparison of reduced dose tacrolimus vs reduced dose ciclosporin can be made, severely limiting the interpretation of the study. Nonetheless the authors were able to demonstrate that ciclosporin at lowered trough concentration enabled all patients clear BKPyV DNAaemia at a mean of 2.7 +/- 2.0 months, although viruria persisted in the majority [75]. This is a shorter time to BKPyV DNAaemia clearance than in other studies undertaking a similar approach, which may be due to varying definitions of the threshold used to define viral clearance [51,131,158]. The mean time to clear SV40 staining on biopsy was 10.7 months (range 8.2 – 21.6 months) and this occurred in 42% of patients; with tacrolimus reduction in the intensity of the SV40 staining noted in the remaining patients [75]. Creatinine was stable in all patients with no graft losses in the 24 month follow-up period [75]. Two patients experienced rejection but both were noted to have been non-compliant with their medications as prescribed [75].
Brennan et al carried out a prospective study of 200 adult patients with reduction in immunosuppression at the point of confirmation of BKPyV DNAaemia, achieved by discontinuation of azathioprine or MMF and reduction in CNI levels after four weeks if DNAaemia persisted [76]. They used slightly higher trough CNI levels than Hirsch et al (targeting tacrolimus 3-5ng/ml and ciclosporin 100-200ng/ml) but were able to demonstrate clearance of DNAaemia in 95% of patients by one year with a mean time to clearance of 54 days (range 7-213 days) [59,76]. There was no clear clinical difference in the tacrolimus vs ciclosporin groups, though more viruria was seen in the tacrolimus group. Only 21% of patients achieved clearance of BKPyV viruria [76]. Biopsies were only performed on a ‘for cause’ basis and hence may have missed some subclinical BKVAN but the authors suggested that given the resolution of DNAaemia and stable creatinine, this was unlikely [76].
Reduce CNIs First?
Hassig et al favoured minimisation of CNI and reduction or withdrawal of corticosteroids with secondary reduction in anti-metabolite therapy by 20-25% [129], as did Baek et al [146] and Bischof et al [62]. Ginervi et al suggested a CNI reduction of 15-20% [105]. It should be noted however that Baek et al noted a higher chance of biopsy-proven acute rejection if CNI levels were reduced by greater than 20% [146]. This did not differ if tacrolimus or ciclosporin was used. Hassig et al did not specifically report resolution rates with CNI minimisation but described no differences in the incidence of BKPyV viraemia/BKVAN between ciclosporin and tacrolimus based regimes although they did comment that high cumulative doses of corticosteroids in the ciclosporin group may have masked any difference between the two, noting that Hirsch et al and Kim et al reported an association of BKVAN with cumulative corticosteroid exposure [50,129,159].
The combination of tacrolimus and MMF was reported by Mengel et al to provide a 13 times higher odds ratio of the development of BKVAN in comparison to those regimes using only one of these agents [143]. This may be due to the observation that systemic exposure to the active metabolite of MMF, mycophenolic acid (MPA), is up to two-fold higher in tacrolimus compared to ciclosporin treated patients due to ciclosporin’s inhibition of the enterohepatic recirculation of the major metabolite, MPA glucuronide [160]. Some authors have found an association with use of MMF [125] but others have not [76].
mTOR Inhibitor (mTORi) Use
Some authors support a change from an antiproliferative agent to an mTORi (in conjunction with ciclosporin) if viral loads do not respond to immunosuppression reduction [131,153,155,161,162]. This approach is based on a registry analysis by Dharnidharka et al who found that incidence of BKVAN was substantially lower if mTORi were used [155]. This observation has been confirmed in small observational studies [131,161–164]. Hirsch et al recommended trough sirolimus levels of <6 ng/ml [59]. Jacobi et al used trough mTORi levels of 5-8 ng/ml [131]. Sánchez Fructuoso et al used either sirolimus or everolimus but did not specify the target levels used [161]. Tedesco-Silav et al reported a lower incidence of BKPyV viraemia when using higher levels of everolimus (6-12 vs. 3-8 ng/ml) [165]. Polanco et al [164] and Al-Raisi [124] found benefit of converting CNI to mTORi and continuing MMF.
mTORi are known to have antiviral properties against CMV and hepatitis C [137,166]. It has been proposed that mTORi are advantageous in BKVAN as they do not appear to limit the effectiveness of BKPyV-specific T cells in vitro [137]. An animal model showed that mTORi can increase the antiviral memory of CD8 T-cell differentiation [166]. Conversely, a case report by Hirsch et al described a patient who only cleared BKPyV viraemia after sirolimus was discontinued [167]. However, it is important to note that the patient was highly immunosuppressed with trough ciclosporin levels of 242 ng/ml and 15 mg prednisolone daily and no attempt was made in this case to minimise these first [167]. Radtke et al also demonstrated no beneficial effect of using everolimus for the risk of developing BKPyV DNAaemia [130].
Corticosteroids
High dose (cumulative intravenous methylprednisolone of ≥2 g/month) has been associated with decline in graft function when managing BKPyV DNAaemia [159]. However, there are very few studies looking directly at the specific effects of modulating the steroid component of immunosuppression. Improved outcomes have been reported by reducing other components of immunosuppression whilst maintaining prednisone at 5-10 mg/day [168]. Patients that have inflammation as a result of rejection may explain reports of improvement with steroid use.
In the setting of tubulitis escalated doses of steroids are used but the evidence is still equivocal as to their benefit [169].
References
2. Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Çuburu N, Buck CB. BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J Virol. 2013;87: 10105–10113.
10. Mentzer AJ, Brenner N, Allen N, Littlejohns TJ, Chong AY, Cortes A, et al. Identification of host-pathogen-disease relationships using a scalable multiplex serology platform in UK Biobank. Nat Commun. 2022;13: 1818.
40. Dadhania D, Snopkowski C, Ding R, Muthukumar T, Chang C, Aull M, et al. Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation. 2008;86: 521–528.
50. Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13: 136–145.
51. Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10: 2615–2623.
59. Hirsch HH, Randhawa PS, AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33: e13528.
62. Bischof N, Hirsch HH, Wehmeier C, Amico P, Dickenmann M, Hirt-Minkowski P, et al. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: long-term outcomes. Nephrol Dial Transplant. 2018;34: 1240–1250.
75. Chen X-T, Li J, Deng R-H, Yang S-C, Chen Y-Y, Chen P-S, et al. The therapeutic effect of switching from tacrolimus to low-dose cyclosporine A in renal transplant recipients with BK virus nephropathy. Biosci Rep. 2019;39. doi:10.1042/BSR20182058
76. Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5: 582–594.
77. Baker SC, Mason AS, Slip RG, Skinner KT, Macdonald A, Masood O, et al. Induction of APOBEC3-mediated genomic damage in urothelium implicates BK polyomavirus (BKPyV) as a hit-and-run driver for bladder cancer. Oncogene. 2022;41: 2139–2151.
88. Rahimi Z, Yaghobi R, Afshari A, Roozbeh J, Mokhtari MJ, Hosseini AM. The effect of BKV reactivation on cytokines behavior in kidney transplanted patients. BMC Nephrol. 2022;23: 20.
89. Rau S, Schönermarck U, Jäger G, Stangl M, Guba M, Meiser B, et al. BK virus-associated nephropathy: neutrophil gelatinase-associated lipocalin as a new diagnostic tool? Clin Transplant. 2013;27: E184–91.
90. Yamanaka K, Oka K, Nakazawa S, Hirai T, Kishikawa H, Nishimura K, et al. Immunohistochemical features of BK virus nephropathy in renal transplant recipients. Clin Transplant. 2012;26 Suppl 24: 20–24.
91. Bouatou Y, Stokman G, Claessen N, Roelofs JJTH, Bemelman F, Kers J, et al. Aryl hydrocarbon receptor expression by macrophages and lymphocytes within infiltrates in BK polyomavirus associated nephropathy. Transpl Immunol. 2018;47: 18–21.
92. Pende D, Falco M, Vitale M, Cantoni C, Vitale C, Munari E, et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front Immunol. 2019;10: 1179.
93. Trydzenskaya H, Juerchott K, Lachmann N, Kotsch K, Kunert K, Weist B, et al. The genetic predisposition of natural killer cell to BK virus-associated nephropathy in renal transplant patients. Kidney Int. 2013;84: 359–365.
94. Bauman Y, Nachmani D, Vitenshtein A, Tsukerman P, Drayman N, Stern-Ginossar N, et al. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe. 2011;9: 93–102.
95. Weist BJD, Schmueck M, Fuehrer H, Sattler A, Reinke P, Babel N. The role of CD4(+) T cells in BKV-specific T cell immunity. Med Microbiol Immunol. 2014;203: 395–408.
96. Li Y-J, Wang J-W, Wu H-H, Wang H-H, Chiang Y-J, Yang H-Y, et al. Tumor necrosis factor-alpha blockade suppresses BK polyomavirus replication. Infection. 2023;51: 967–980.
97. Abend JR, Low JA, Imperiale MJ. Inhibitory effect of gamma interferon on BK virus gene expression and replication. J Virol. 2007;81: 272–279.
98. Wu H-H, Li Y-J, Weng C-H, Hsu H-H, Chang M-Y, Yang H-Y, et al. Interferon-alpha and MxA inhibit BK polyomavirus replication by interaction with polyomavirus large T antigen. Biomed J. 2023; 100682.
99. Soto JA, Gálvez NMS, Andrade CA, Pacheco GA, Bohmwald K, Berrios RV, et al. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front Immunol. 2020;11: 1513.
100. Womer KL, Huang Y, Herren H, Dibadj K, Peng R, Murawski M, et al. Dendritic cell deficiency associated with development of BK viremia and nephropathy in renal transplant recipients. Transplantation. 2010;89: 115–123.
101. Drake DR 3rd, Shawver ML, Hadley A, Butz E, Maliszewski C, Lukacher AE. Induction of polyomavirus-specific CD8(+) T lymphocytes by distinct dendritic cell subpopulations. J Virol. 2001;75: 544–547.
102. Drake DR 3rd, Moser JM, Hadley A, Altman JD, Maliszewski C, Butz E, et al. Polyomavirus-infected dendritic cells induce antiviral CD8(+) T lymphocytes. J Virol. 2000;74: 4093–4101.
103. Gedvilaite A, Dorn DC, Sasnauskas K, Pecher G, Bulavaite A, Lawatscheck R, et al. Virus-like particles derived from major capsid protein VP1 of different polyomaviruses differ in their ability to induce maturation in human dendritic cells. Virology. 2006;354: 252–260.
104. Randhawa PS, Gupta G, Vats A, Shapiro R, Viscidi RP. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin Vaccine Immunol. 2006;13: 1057–1063.
105. Ginevri F, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7: 2727–2735.
106. Schachtner T, Müller K, Stein M, Diezemann C, Sefrin A, Babel N, et al. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant. 2011;11: 2443–2452.
107. Schaenman JM, Korin Y, Sidwell T, Kandarian F, Harre N, Gjertson D, et al. Increased Frequency of BK Virus-Specific Polyfunctional CD8+ T Cells Predict Successful Control of BK Viremia After Kidney Transplantation. Transplantation. 2017;101: 1479–1487.
108. Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis. 2007;9: 165–170.
109. Renner FC, Dietrich H, Bulut N, Celik D, Freitag E, Gaertner N, et al. The risk of polyomavirus-associated graft nephropathy is increased by a combined suppression of CD8 and CD4 cell-dependent immune effects. Transplant Proc. 2013;45: 1608–1610.
110. Zhou W, Sharma M, Martinez J, Srivastava T, Diamond DJ, Knowles W, et al. Functional characterization of BK virus-specific CD4+ T cells with cytotoxic potential in seropositive adults. Viral Immunol. 2007;20: 379–388.
111. Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435: 682–686.
112. Chen Y, Trofe J, Gordon J, Du Pasquier RA, Roy-Chaudhury P, Kuroda MJ, et al. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80: 3495–3505.
113. Hammer MH, Brestrich G, Andree H, Engelmann E, Rosenberger C, Tillmann H, et al. HLA type-independent method to monitor polyoma BK virus-specific CD4 and CD8 T-cell immunity. Am J Transplant. 2006;6: 625–631.
114. Randhawa PS, Popescu I, Macedo C, Zeevi A, Shapiro R, Vats AN, et al. Detection of CD8+ T cells sensitized to BK virus large T antigen in healthy volunteers and kidney transplant recipients. Hum Immunol. 2006;67: 298–302.
115. Schneidawind D, Schmitt A, Wiesneth M, Mertens T, Bunjes D, Freund M, et al. Polyomavirus BK-specific CD8+ T cell responses in patients after allogeneic stem cell transplant. Leuk Lymphoma. 2010;51: 1055–1062.
116. Leuenberger D, Andresen PA, Gosert R, Binggeli S, Ström EH, Bodaghi S, et al. Human polyomavirus type 1 (BK virus) agnoprotein is abundantly expressed but immunologically ignored. Clin Vaccine Immunol. 2007;14: 959–968.
117. Binggeli S, Egli A, Schaub S, Binet I, Mayr M, Steiger J, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7: 1131–1139.
118. Mueller K, Schachtner T, Sattler A, Meier S, Friedrich P, Trydzenskaya H, et al. BK-VP3 as a new target of cellular immunity in BK virus infection. Transplantation. 2011;91: 100–107.
119. Zeng G, Huang Y, Huang Y, Lyu Z, Lesniak D, Randhawa P. Antigen-Specificity of T Cell Infiltrates in Biopsies With T Cell-Mediated Rejection and BK Polyomavirus Viremia: Analysis by Next Generation Sequencing. Am J Transplant. 2016;16: 3131–3138.
120. Cioni M, Leboeuf C, Comoli P, Ginevri F, Hirsch HH. Characterization of Immunodominant BK Polyomavirus 9mer Epitope T Cell Responses. Am J Transplant. 2016;16: 1193–1206.
121. Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, et al. Prevalence of Polyomavirus BK and JC Infection and Replication in 400 Healthy Blood Donors. J Infect Dis. 2009;199: 837–846.
122. Krymskaya L, Sharma MC, Martinez J, Haq W, Huang EC, Limaye AP, et al. Cross-reactivity of T lymphocytes recognizing a human cytotoxic T-lymphocyte epitope within BK and JC virus VP1 polypeptides. J Virol. 2005;79: 11170–11178.
123. Ambalathingal GR, Francis RS, Corvino D, Srihari S, Aftab BT, Smith C, et al. Proteome-wide analysis of T-cell response to BK polyomavirus in healthy virus carriers and kidney transplant recipients reveals a unique transcriptional and functional profile. Clin Transl Immunology. 2020;9: e01102.
124. Al-Raisi F, Mohsin N, Kamble P. Management of BK virus nephropathy in kidney transplant recipients at the Royal Hospital – Clinical Audit – Oman. Exp Clin Transplant. 2015;13 Suppl 1: 156–158.
125. Elfadawy N, Flechner SM, Liu X, Schold J, Tian D, Srinivas TR, et al. The impact of surveillance and rapid reduction in immunosuppression to control BK virus-related graft injury in kidney transplantation. Transpl Int. 2013;26: 822–832.
126. White LH, Casian A, Hilton R, Macphee IAM, Marsh J, Sweny P, et al. BK virus nephropathy in renal transplant patients in London. Transplantation. 2008;85: 1008–1015.
127. Fichtner A, Schmidt J, Süsal C, Carraro A, Oh J, Zirngibl M, et al. Risk of cellular or antibody-mediated rejection in pediatric kidney transplant recipients with BK polyomavirus replication-an international CERTAIN registry study. Pediatr Nephrol. 2024. doi:10.1007/s00467-024-06501-7
128. Bayraktar A, Catma Y, Akyildiz A, Demir E, Bakkaloglu H, Ucar AR, et al. Infectious Complications of Induction Therapies in Kidney Transplantation. Ann Transplant. 2019;24: 412–417.
129. Hässig A, Roos M, Etter A, Bossart W, Müller N, Schiesser M, et al. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl Infect Dis. 2014;16: 44–54.
130. Radtke J, Dietze N, Fischer L, Achilles E-G, Li J, Scheidat S, et al. Incidence of BK polyomavirus infection after kidney transplantation is independent of type of immunosuppressive therapy. Transpl Infect Dis. 2016;18: 850–855.
131. Jacobi J, Prignitz A, Büttner M, Korn K, Weidemann A, Hilgers KF, et al. BK viremia and polyomavirus nephropathy in 352 kidney transplants; risk factors and potential role of mTOR inhibition. BMC Nephrol. 2013;14: 207.
132. Saull HE, Enderby CY, Gonwa TA, Wadei HM. Comparison of alemtuzumab vs. antithymocyte globulin induction therapy in primary non-sensitized renal transplant patients treated with rapid steroid withdrawal. Clin Transplant. 2015;29: 573–580.
133. Welberry Smith MP, Cherukuri A, Newstead CG, Lewington AJP, Ahmad N, Menon K, et al. Alemtuzumab induction in renal transplantation permits safe steroid avoidance with tacrolimus monotherapy: a randomized controlled trial. Transplantation. 2013;96: 1082–1088.
134. Chan K, Taube D, Roufosse C, Cook T, Brookes P, Goodall D, et al. Kidney transplantation with minimized maintenance: alemtuzumab induction with tacrolimus monotherapy–an open label, randomized trial. Transplantation. 2011;92: 774–780.
135. Theodoropoulos N, Wang E, Penugonda S, Ladner DP, Stosor V, Leventhal J, et al. BK virus replication and nephropathy after alemtuzumab-induced kidney transplantation. Am J Transplant. 2013;13: 197–206.
136. Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89: 1057–1070.
137. Egli A, Köhli S, Dickenmann M, Hirsch HH. Inhibition of polyomavirus BK-specific T-Cell responses by immunosuppressive drugs. Transplantation. 2009;88: 1161–1168.
138. Hârza M, Tacu D, Mitroi G, Bucşa C, Gherghiceanu M, Preda A, et al. Polyomavirus BK-associated nephropathy after kidney transplantation: a single-center retrospective analysis. Rom J Morphol Embryol. 2014;55: 123–128.
139. Howell DN, Smith SR, Butterly DW, Klassen PS, Krigman HR, Burchette JL Jr, et al. Diagnosis and management of BK polyomavirus interstitial nephritis in renal transplant recipients. Transplantation. 1999;68: 1279–1288.
140. Sood P, Senanayake S, Sujeet K, Medipalli R, Zhu YR, Johnson CP, et al. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94: 814–821.
141. Saad ER, Bresnahan BA, Cohen EP, Lu N, Orentas RJ, Vasudev B, et al. Successful treatment of BK viremia using reduction in immunosuppression without antiviral therapy. Transplantation. 2008;85: 850–854.
142. Wu C, Randhawa P, McCauley J. Transplantation: polyomavirus nephropathy and the risk of specific immunosuppression regimens. ScientificWorldJournal. 2006;6: 512–528.
143. Mengel M, Marwedel M, Radermacher J, Eden G, Schwarz A, Haller H, et al. Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant. 2003;18: 1190–1196.
144. Lipshutz GS, Flechner SM, Govani MV, Vincenti F. BK nephropathy in kidney transplant recipients treated with a calcineurin inhibitor-free immunosuppression regimen. Am J Transplant. 2004;4: 2132–2134.
145. Vincenti F. Immunosuppression minimization: current and future trends in transplant immunosuppression. J Am Soc Nephrol. 2003;14: 1940–1948.
146. Baek CH, Kim H, Yu H, Yang WS, Han DJ, Park S-K. Risk Factors of Acute Rejection in Patients with BK Nephropathy After Reduction of Immunosuppression. Ann Transplant. 2018;23: 704–712.
147. Gard L, van Doesum W, Niesters HGM, van Son WJ, Diepstra A, Stegeman CA, et al. A delicate balance between rejection and BK polyomavirus associated nephropathy; A retrospective cohort study in renal transplant recipients. PLoS One. 2017;12: e0178801.
148. Kayler LK, Mohanka R, Morgan C, Basu A, Shapiro R, Randhawa PS. Clinical course of kidney transplant patients with acute rejection and BK virus replication following Campath therapy. Clin Transplant. 2008;22: 348–353.
149. Gheith O, Al-Otaibi T, Elserwy N, Elsawy I, Donia F, Fathi T, et al. Reversible Ischemic Nephropathy in a Deceased Donor Renal Transplant Recipient With BK Nephropathy. Exp Clin Transplant. 2022;20: 132–135.
150. Ott U, Steiner T, Busch M, Gerth J, Wolf G. A single-center experience with BK virus nephropathy. Clin Nephrol. 2008;69: 244–250.
151. Abdeltawab K, Denewar A, Gheith O, Zein Eldin S, Yagen J, AbdelMonem M, et al. Successful Management of Combined BK Nephropathy and Nocardiosis in a Renal Transplant Recipient: Case Report. Exp Clin Transplant. 2022;20: 140–144.
152. Takayama T, Ito T, Suzuki K, Ushiyama T, Horii T, Miura K, et al. BK virus nephropathy: clinical experience in a university hospital in Japan. Int J Urol. 2009;16: 924–928.
153. Gouvêa ALF, Cosendey RIJ, Nascimento ALR, Carvalho FR, Silva AA, de Moraes HP, et al. BK polyomavirus nephropathy in two kidney transplant patients with distinct diagnostic strategies for BK virus and similar clinical outcomes: two case reports. J Med Case Rep. 2017;11: 146.
154. Bressollette-Bodin C, Coste-Burel M, Hourmant M, Sebille V, Andre-Garnier E, Imbert-Marcille BM. A prospective longitudinal study of BK virus infection in 104 renal transplant recipients. Am J Transplant. 2005;5: 1926–1933.
155. Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87: 1019–1026.
156. Kizilbash SJ, Rheault MN, Bangdiwala A, Matas A, Chinnakotla S, Chavers BM. Infection rates in tacrolimus versus cyclosporine-treated pediatric kidney transplant recipients on a rapid discontinuation of prednisone protocol: 1-year analysis. Pediatr Transplant. 2017;21. doi:10.1111/petr.12919
157. Li Y-J, Weng C-H, Lai W-C, Wu H-H, Chen Y-C, Hung C-C, et al. A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus. Transplantation. 2010;89: 299–306.
158. Kable K, Davies CD, O’connell PJ, Chapman JR, Nankivell BJ. Clearance of BK Virus Nephropathy by Combination Antiviral Therapy With Intravenous Immunoglobulin. Transplant Direct. 2017;3: e142.
159. Kim H, Yu H, Baek CH, Han DJ, Park S-K. High-dose steroid therapy in BK viremia adversely affected the long-term graft function after kidney transplantation. Transpl Infect Dis. 2016;18: 844–849.
160. van Gelder T, Klupp J, Barten MJ, Christians U, Morris RE. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit. 2001;23: 119–128.
161. Sánchez Fructuoso AI, Calvo N, Perez-Flores I, Valero R, Rodríguez-Sánchez B, García de Viedma D, et al. Mammalian target of rapamycin signal inhibitors could play a role in the treatment of BK polyomavirus nephritis in renal allograft recipients. Transpl Infect Dis. 2011;13: 584–591.
162. Wali RK, Drachenberg C, Hirsch HH, Papadimitriou J, Nahar A, Mohanlal V, et al. BK virus-associated nephropathy in renal allograft recipients: rescue therapy by sirolimus-based immunosuppression. Transplantation. 2004;78: 1069–1073.
163. Benavides CA, Pollard VB, Mauiyyedi S, Podder H, Knight R, Kahan BD. BK virus-associated nephropathy in sirolimus-treated renal transplant patients: incidence, course, and clinical outcomes. Transplantation. 2007;84: 83–88.
164. Polanco N, González Monte E, Folgueira MD, Morales E, Gutiérrez Martínez E, Bengoa I, et al. Everolimus-based immunosuppression therapy for BK virus nephropathy. Transplant Proc. 2015;47: 57–61.
165. Tedesco Silva H Jr, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, et al. Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant. 2010;10: 1401–1413.
166. Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460: 108–112.
167. Hirsch HH, Mohaupt M, Klimkait T. Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J Infect Dis. 2001;184: 1494–5; author reply 1495–6.
168. Weiss AS, Gralla J, Chan L, Klem P, Wiseman AC. Aggressive Immunosuppression Minimization Reduces Graft Loss Following Diagnosis of BK Virus-Associated Nephropathy: A Comparison of Two Reduction Strategies. Clin J Am Soc Nephrol. 2008;3: 1812.
169. Carrillo J, Del Bello A, Sallusto F, Delas A, Colombat M, Mansuy JM, et al. Effect of steroid pulses in severe BK virus allograft nephropathy with extensive interstitial inflammation. Transpl Infect Dis. 2024;26. doi:10.1111/tid.14260
4. Treatment of BKPyV Infection
Based on current evidence, the panel cannot recommend any agent as an isolated treatment for managing BKVAN or DNAaemia. There are no treatments with robust efficacy data, except for reduction in immunosuppression (discussed in section 3) and none currently have NICE recommendation. The agents listed below ought only be additive to reducing the immunosuppressant burden but their value is so far unproven. Whilst acknowledging that the following interventions have been previously evaluated under certain clinical conditions (such as treating resistant infections), we cannot present them as recommended therapies. Additionally, where such therapies are introduced; patient tolerance in relation to hepatic function, renal impairment, the elderly and the very young is important.
Many of the agents listed below showed potentially promising results during in vitro evaluation that have not translated into clinical efficacy. It is noteworthy that in the majority of the available evidence, many interventions were tried simultaneously or sequentially which makes it harder to determine any specific effect of individual actions or medications. Randomised controlled trials are, therefore, a priority.
Therapeutic interventions that have been tested clinically for managing BKVAN or DNAaemia but cannot be recommended based on current evidence levels are listed below:
- Leflunomide is undergoing phase 2/3 trials for the treatment of BKPyV in the US; however, there are also small reports which have failed to show efficacy.
- Cidofovir has equivocal clinical efficacy data and a clear risk of nephrotoxicity.
- Brincidofovir is currently being evaluated in an Australian/Japanese clinical trial (NCT05511779 BCV-BN01) recruitment is expected to complete in February 2025 there is no evidence to support its use at this time.
- Fluroquinolone Antibiotics (levofloxacin and ciprofloxacin) have been evaluated in randomised controlled trials with no evidence of efficacy
- Intravenous Immunoglobulin (IVIg) has been trialled with little support for efficacy, a national audit of its use is underway and is not NICE-approved.
- A recently completed clinical trial of a monoclonal antibody therapy targeted against BKPyV “MAU868” has demonstrated safety as a first-in-class therapeutic agent; however, efficacy data is still in development.
- T cell therapies
- BKPyV-specific T cells are in early phases of development and have not reached the point where it can be recommended as a form of treatment and they have not progressed into randomised controlled trials.
- Posoleucel is an allogeneic, off-the-shelf multivirus-specific T-Cell therapy that performed well in its first phase II trial directed at haemorrhagic cystitis, however ongoing clinical trials are delivering mixed results.
- ExtraCorporeal Photopheresis (ECP) is an immunomodulatory therapy targeting T cells isolated from the patient and inducing apoptosis in them before returning them to the patient. There is very limited data on ECP to-date.
- Mirozibine has been trialled as a replacement for MMF but the positive effects may however be due to reduced immunosuppressant burden and trials are ongoing.
Research recommendations:
- The development of effective BKPyV-specific antiviral agents, underpinned by improvements in scientific understanding of BKPyV, is a critical research priority.
- Multi-centre and multi-arm randomised controlled trials to evaluate clinical efficacy of currently used and novel agents
- What is the clinical- and cost-effectiveness of using a BKPyV DNAaemia load threshold to guide cessation of treatment?
- Evaluation of preventive/prophylactic strategies, such as vaccination, in at-risk recipients (for example the BKPyV seronegative).
Audit Measures:
- Assessment of outcomes, including complications, of current agents in clinical use for intervention post immunosuppression reduction.
- Patient tolerance, dosing, monitoring and completion of adjuvant therapies.
Rationale
Various medications have been suggested as treatment options for BKPyV. It is difficult to determine how much of the reported effect of these interventions are due to reduction in immunosuppression or due to direct antiviral effects, as noted by a systematic review [136] and recent Cochrane review [170]. The key approaches tested to-date are detailed in turn below. Almost all studies on treatment are limited by follow-up periods that are not sufficient to robustly inform clinicians on the longer-term impacts on graft survival in either over- or under- immunosuppression in the context of detectable BKPyV/BKVAN.
Leflunomide
Leflunomide is clinically considered an immunomodulatory drug because its mechanism of action is inhibition of both tyrosine kinases and mitochondrial -dihydroorotate dehydrogenase, which synthesises uridine monophosphate, an essential precursor for DNA and RNA [171–173]. This inhibition of DNA synthesis slows the cell division of rapidly dividing cells like lymphocytes [174], but also inhibits viral genome replication in vitro leading to the use of leflunomide against BKPyV [175–177]. The dual antiviral and immunosuppressant activity made the concept of this agent’s role attractive. Though we are not recommending its use in this guideline it remains an area of ongoing research.
Leflunomide use was described by Ott et al [150] and Al-Raisi et al [124] to reasonable effect. This is in contrast to other studies reporting a lack of efficacy both for leflunomide [125,178] and FK778, that is derived from a leflunomide metabolite [179]. Pharmacodynamic analysis of A77 1726, which is an active metabolite of leflunomide, suggested an association between A77 1726 concentrations and BKPyV DNAaemia [180]but a more detailed analysis could not find this association [178].
Williams et al replaced MMF with leflunomide (alongside continuing corticosteroids and tacrolimus with trough levels of 4-6 ng/ml) in 17 patients with all achieving either complete clearance of viraemia or a progressive significant reduction in viral levels [180]. Jaw et al describe the substitution of MMF with leflunomide if BKPyV DNAaemia failed to respond within three months to reduction in immunosuppression with the addition of everolimus (trough target level 5 ng/ml) between one to five months later. In the three cases that they describe, one had complete resolution of DNAaemia and two had significant viral load reductions [181].
An in vitro study suggested a synergistic effect between leflunomide and mTOR inhibition [182] which was followed up in a case study where the combination was used to support the eradication of HHV8 in a renal transplant recipient with Kaposi’s sarcoma [183], but this approach has not been attempted against BKPyV.
It should be noted that leflunomide has the potential for significant hepatotoxicity and/or haematological issues and there is wide variability in interpatient metabolism and therefore, tolerability [184,185] without measuring levels. Williams et al proposed the need for monitoring of the active metabolite and suggested levels above 40 µg/ml were more likely to demonstrate efficacy against BKPyV [180]. In their series, only one patient required dose adjustment and none had any serious adverse effects [180]. Josephson et al observed similar results [186].
It is of note that leflunomide’s mechanism against CMV is thought to be through preventing tegument acquisition by viral nucleocapsids. Since BKPyV is a non-enveloped virus, results from CMV studies are not likely to be relevant to BKPyV [187].
Cidofovir
Cidofovir has been proposed as an alternative antiviral agent in various case studies reporting possible benefit [124,129,151,175,188–190] but inconsistently [125]. However, cidofovir has been demonstrated to be nephrotoxic at doses required to achieve any effect on BKPyV DNAaemia [64,191–193]. Cidofovir can cause proteinuria (seen in 40% cases [194]). Interstitial nephritis has been described [191] and progressive renal dysfunction, including end stage renal failure [194,195]. Proximal tubular acidosis is also commonly seen with treatment [194] and anterior uveitis was described in 12-35% cases [189,196]. Kupyers et al used a low-dose cidofovir regime in a small cohort of eight patients with BKVAN and demonstrated no significant side effects [190]. A meta-analysis by Johnston et al showed no benefit in the addition of cidofovir or leflunomide to graft survival above reduction in immunosuppression [136].
Brincidofovir is a prodrug formulation designed to release cidofovir inside cells with the aim of reducing plasma concentrations of cidofovir and reducing side effects. Brincidofovir has an active Australian/Japanese clinical trial (NCT05511779 BCV-BN01), but there is no published evidence to support its use at this time.
Fluroquinolone Antibiotics
Fluoroquinolone antibiotics have also been proposed as a treatment for BKPyV DNAaemia after observational series noted a lower incidence of BKVAN in patients taking these for another reason [197–200]. In vitro antiviral activity has also been observed [197–199]. There are two prospective RCTs involving the use of levofloxacin [201,202] and one using ciprofloxacin [203]; however, none showed any benefit in the prevention of BKPyV DNAaemia. Another small prospective observational study by Elfadawy et al of ciprofloxacin for the treatment of BKPyV DNAaemia identified no value to its use [125].
Intravenous Immunoglobulin
Intravenous immunoglobulin (IVIg) is commonly tried if patients with BKVAN do not respond to a reduction in immunosuppression [124,129,151,153,181,204,205] with the intended benefit of deriving an improved immune response, potentially through an immunomodulatory effect [206] or by direct neutralising anti-BKPyV activity [206–208]. In the UK, IVIg is often given at a dose of 300 mg/kg every three weeks in conjunction with a reduction in immunosuppression with the goal of maintaining an IgG level >400 mg/dL. In some centres IVIg is given at larger doses with longer intervals; however, there is no RCT support for either approach. IVIg is typically administered alongside intravenous hydration to mitigate the risk of acute kidney injury (AKI). Studies have been contradictory with benefit reported by some [158,204,205,209–212] but not all [32,181]. Most studies also report using additional antiviral treatments alongside IVIg which makes interpretation of benefit challenging [158,181,205,209–212]. Benotmane et al observed that newly-transplanted patients treated with IVIg had lower viral titres at twelve months than those who were not treated and suggested that this may have prevented viral replication [212].
The effectiveness of BKPyV-specific monoclonal antibodies are currently being assessed in ongoing trials. A recently completed clinical trial of a human IgG1 monoclonal neutralising antibody therapy targeted against BKPyV “MAU868” found it was safe and well-tolerated [213]. Whilst results to-date show promise in relation to safety and ex vivo neutralising activity, we are still waiting for clinical efficacy data relating to MAU868 [213].
T cell therapies
T cell adoptive therapy, where BKPyV-reactive T cells are generated ex vivo and then infused, has been reported in a single case study [214]. Although viral clearance was demonstrated, the graft was lost and it was proposed that this was due to the late inclusion of this treatment strategy as the patient had previously failed to respond to all of the other management strategies outlined above [214]. The authors suggested that the resultant fibrosis of the graft was by this point too advanced to achieve any clinical benefit [214]. There is a multicentre trial ongoing using this therapy after positive results were seen in the development of BKPyV-associated haemorrhagic cystitis or nephropathy in hematopoietic cell transplant recipients (ClinicalTrials.gov identifier: NCT04605484).
Posoleucel is an allogeneic, off-the-shelf multivirus-specific T cell therapy that recently had its first phase II trial of 23 patients with BKPyV hemorrhagic cystitis where complete resolution of macroscopic hematuria and symptoms was observed in 74% at 6 weeks post-treatment [215]. However the study only included 2 patients with BKVAN and little effect was observed. [215]. More recent phase 3 trials of the antiviral following allogeneic hematopoietic cell transplant were abandoned due to lack of efficacy at interim analyses [216]. A recently published Phase II trial of posoleucel in renal transplant recipients has shown efficacy in reducing BKPyV DNAaemia [217]. Posoleucel has shown no safety concerns in trials to-date and there may be more research to come in renal transplant recipients [216,217].
ExtraCorporeal Photopheresis (ECP) is an immunomodulatory therapy targeting T cells isolated from the patient and inducing apoptosis in them before returning them to the patient. The treatments given to the isolated cells outside of the body vary but UVA and radiation have been used to induce apoptosis (reviewed [218]). There is very limited data on the use of ECP to treat BKPyV to-date [219,220] but this is a research-active area.
Mizoribine
Mizoribine, an imidazole nucleoside analogue, investigated by Yuan et al [221], Funshashi et al [222] and Li et al [223], all of whom substituted MMF for mizoribine in small case series. The effect may however be due to reduced immunosuppressant burden. Mizoribine is an immunosuppressive agent which acts in a similar fashion to MMF by inhibiting cellular and humoral immune responses by blocking inosine 5-monophosphate dehydrogenase, which is a rate-limiting enzyme for de novo purine synthesis and critical for the proliferation of T and B lymphocytes [223,224]. Mizoribine is renally cleared and so requires dose-adjustment for graft function [223,224]. Hyperuricaemia is a noted common side effect [221–223] but this responds to standard treatment with febuxostat. The latter can however be associated with renal impairment hence caution is required. A clinical trial is scheduled to assess the potential for efficacy using mizoribine in larger numbers of patients (ClinicalTrials.gov Identifier: NCT05293704).
The need for better research models
Great progress has been made in our understanding of the molecular virology of BKPyV over recent decades e.g. identification of the virus microRNA and identification of key host factors necessary for the infection process. Despite this, gaps remain in our knowledge hindering our ability to develop targeted approaches against BKPyV. In particular, BKPyV research has been hampered by a lack of sophisticated cellular models to enable analysis of virus infection in close to physiological relevant situations. Tubular epithelial cells are tightly connected and have unique apical and basolateral membranes, with highly specialised functions; however, most in vitro BKPyV life cycle research has been performed in non-polarised monkey kidney cell lines (e.g. [225]) and more recently in undifferentiated primary human renal proximal tubular epithelial (RPTE) cells [226]. Recent attempts have been made to study BKPyV replication in polarised RPTE cells (e.g. [227]); however, to more faithfully recapitulate a physiologically relevant infection, researchers must capitalise on the recent development of three-dimensional multicellular organoids to understand how BKPyV disseminates throughout the reno-urinary tract to cause damage. Complex kidney organoids representing multiple cell types and structures can be generated from iPSC [228], and more recently patient-derived primary renal tubular epithelial organoids “tubuloids” have been produced, which represent distinct tubule structures but lack glomerular cells. Crucially, these tubuloids can be infected with BKPyV [229]. BKPyV research may also benefit in the future from newer kidney organoid – immune cell co-cultures able to model the complex interactions between kidney epithelial cells and cells of the immune system, which likely play roles in both viral ‘latency’ and pathology.
References
32. Wadei HM, Rule AD, Lewin M, Mahale AS, Khamash HA, Schwab TR, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant. 2006;6: 1025–1032.
64. Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79: 1277–1286.
124. Al-Raisi F, Mohsin N, Kamble P. Management of BK virus nephropathy in kidney transplant recipients at the Royal Hospital – Clinical Audit – Oman. Exp Clin Transplant. 2015;13 Suppl 1: 156–158.
125. Elfadawy N, Flechner SM, Liu X, Schold J, Tian D, Srinivas TR, et al. The impact of surveillance and rapid reduction in immunosuppression to control BK virus-related graft injury in kidney transplantation. Transpl Int. 2013;26: 822–832.
129. Hässig A, Roos M, Etter A, Bossart W, Müller N, Schiesser M, et al. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl Infect Dis. 2014;16: 44–54.
136. Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89: 1057–1070.
150. Ott U, Steiner T, Busch M, Gerth J, Wolf G. A single-center experience with BK virus nephropathy. Clin Nephrol. 2008;69: 244–250.
151. Abdeltawab K, Denewar A, Gheith O, Zein Eldin S, Yagen J, AbdelMonem M, et al. Successful Management of Combined BK Nephropathy and Nocardiosis in a Renal Transplant Recipient: Case Report. Exp Clin Transplant. 2022;20: 140–144.
153. Gouvêa ALF, Cosendey RIJ, Nascimento ALR, Carvalho FR, Silva AA, de Moraes HP, et al. BK polyomavirus nephropathy in two kidney transplant patients with distinct diagnostic strategies for BK virus and similar clinical outcomes: two case reports. J Med Case Rep. 2017;11: 146.
158. Kable K, Davies CD, O’connell PJ, Chapman JR, Nankivell BJ. Clearance of BK Virus Nephropathy by Combination Antiviral Therapy With Intravenous Immunoglobulin. Transplant Direct. 2017;3: e142.
170. Wajih Z, Karpe KM, Walters GD. Interventions for BK virus infection in kidney transplant recipients. Cochrane Database Syst Rev. 2024;10: CD013344.
171. Davis JP, Cain GA, Pitts WJ, Magolda RL, Copeland RA. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996;35: 1270–1273.
172. Siemasko K, Chong AS, Jäck HM, Gong H, Williams JW, Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol. 1998;160: 1581–1588.
173. Xu X, Williams JW, Bremer EG, Finnegan A, Chong AS. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem. 1995;270: 12398–12403.
174. Cherwinski HM, McCarley D, Schatzman R, Devens B, Ransom JT. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. J Pharmacol Exp Ther. 1995;272: 460–468.
175. Farasati NA, Shapiro R, Vats A, Randhawa P. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation. 2005;79: 116–118.
176. Jeffers-Francis LK, Burger-Calderon R, Webster-Cyriaque J. Effect of Leflunomide, Cidofovir and Ciprofloxacin on replication of BKPyV in a salivary gland in vitro culture system. Antiviral Res. 2015;118: 46–55.
177. Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol. 2010;84: 2150–2156.
178. Krisl JC, Taber DJ, Pilch N, Chavin K, Bratton C, Thomas B, et al. Leflunomide efficacy and pharmacodynamics for the treatment of BK viral infection. Clin J Am Soc Nephrol. 2012;7: 1003–1009.
179. Guasch A, Roy-Chaudhury P, Woodle ES, Fitzsimmons W, Holman J, First MR, et al. Assessment of efficacy and safety of FK778 in comparison with standard care in renal transplant recipients with untreated BK nephropathy. Transplantation. 2010;90: 891–897.
180. Williams JW, Javaid B, Kadambi PV, Gillen D, Harland R, Thistlewaite JR, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352: 1157–1158.
181. Jaw J, Hill P, Goodman D. Combination of Leflunomide and Everolimus for treatment of BK virus nephropathy. Nephrology . 2017;22: 326–329.
182. Liacini A, Seamone ME, Muruve DA, Tibbles LA. Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus infection. Transplantation. 2010;90: 1450–1457.
183. Basu G, Mohapatra A, Manipadam MT, Mani SE, John GT. Leflunomide with low-dose everolimus for treatment of Kaposi’s sarcoma in a renal allograft recipient. Nephrol Dial Transplant. 2011;26: 3412–3415.
184. Leca N, Muczynski KA, Jefferson JA, de Boer IH, Kowalewska J, Kendrick EA, et al. Higher levels of leflunomide are associated with hemolysis and are not superior to lower levels for BK virus clearance in renal transplant patients. Clin J Am Soc Nephrol. 2008;3: 829–835.
185. Launay M, Baudouin V, Guillemain R, Maisin A, Flodrops H, Douez E, et al. Leflunomide for BKvirus: Report of Seven Kidney-Transplanted Children. Int J Organ Transplant Med. 2018;9: 178–183.
186. Josephson MA, Gillen D, Javaid B, Kadambi P, Meehan S, Foster P, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81: 704–710.
187. Waldman WJ, Knight DA, Lurain NS, Miller DM, Sedmak DD, Williams JW, et al. Novel mechanism of inhibition of cytomegalovirus by the experimental immunosuppressive agent leflunomide. Transplantation. 1999;68: 814–825.
188. Keller LS, Peh CA, Nolan J, Bannister KM, Clarkson AR, Faull RJ. BK transplant nephropathy successfully treated with cidofovir. Nephrol Dial Transplant. 2003;18: 1013–1014.
189. Kuypers DRJ, Bammens B, Claes K, Evenepoel P, Lerut E, Vanrenterghem Y. A single-centre study of adjuvant cidofovir therapy for BK virus interstitial nephritis (BKVIN) in renal allograft recipients. J Antimicrob Chemother. 2009;63: 417–419.
190. Kuypers DRJ, Vandooren A-K, Lerut E, Evenepoel P, Claes K, Snoeck R, et al. Adjuvant low-dose cidofovir therapy for BK polyomavirus interstitial nephritis in renal transplant recipients. Am J Transplant. 2005;5: 1997–2004.
191. Vats A, Shapiro R, Singh Randhawa P, Scantlebury V, Tuzuner A, Saxena M, et al. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation. 2003;75: 105–112.
192. Kadambi PV, Josephson MA, Williams J, Corey L, Jerome KR, Meehan SM, et al. Treatment of refractory BK virus-associated nephropathy with cidofovir. Am J Transplant. 2003;3: 186–191.
193. Coomes EA, Wolfe Jacques A, Michelis FV, Kim DDH, Thyagu S, Viswabandya A, et al. Efficacy of cidofovir in treatment of BK virus-induced hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2018;24: 1901–1905.
194. Bagnis C, Izzdine H, Deray G. [Renal tolerance of cidofovir]. Therapie. 1999;54: 689–691.
195. Vandercam B, Moreau M, Goffin E, Marot JC, Cosyns JP, Jadoul M. Cidofovir-induced end-stage renal failure. Clin Infect Dis. 1999;29: 948–949.
196. Lopez V, Sola E, Gutierrez C, Burgos D, Cabello M, García I, et al. Anterior uveitis associated with treatment with intravenous cidofovir in kidney transplant patients with BK virus nephropathy. Transplant Proc. 2006;38: 2412–2413.
197. Gabardi S, Waikar SS, Martin S, Roberts K, Chen J, Borgi L, et al. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol. 2010;5: 1298–1304.
198. Sharma BN, Li R, Bernhoff E, Gutteberg TJ, Rinaldo CH. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antiviral Res. 2011;92: 115–123.
199. Ali SH, Chandraker A, DeCaprio JA. Inhibition of Simian virus 40 large T antigen helicase activity by fluoroquinolones. Antivir Ther. 2007;12: 1–6.
200. Wojciechowski D, Chanda R, Chandran S, Lee B, Webber A, Macaraig M, et al. Ciprofloxacin prophylaxis in kidney transplant recipients reduces BK virus infection at 3 months but not at 1 year. Transplantation. 2012;94: 1117–1123.
201. Knoll GA, Humar A, Fergusson D, Johnston O, House AA, Kim SJ, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA. 2014;312: 2106–2114.
202. Lee BT, Gabardi S, Grafals M, Hofmann RM, Akalin E, Aljanabi A, et al. Efficacy of levofloxacin in the treatment of BK viremia: a multicenter, double-blinded, randomized, placebo-controlled trial. Clin J Am Soc Nephrol. 2014;9: 583–589.
203. Patel SJ, Knight RJ, Kuten SA, Graviss EA, Nguyen DT, Moore LW, et al. Ciprofloxacin for BK viremia prophylaxis in kidney transplant recipients: Results of a prospective, double-blind, randomized, placebo-controlled trial. Am J Transplant. 2019;19: 1831–1837.
204. Anyaegbu EI, Almond PS, Milligan T, Allen WR, Gharaybeh S, Al-Akash SI. Intravenous immunoglobulin therapy in the treatment of BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr Transplant. 2012;16: E19–24.
205. Hwang SD, Lee JH, Lee SW, Kim JK, Kim M-J, Song JH. High-Dose Intravenous Immunoglobulin Treatment of Polyomavirus Nephropathy Developing After T Cell-Mediated Rejection Treatment: A Case Report. Transplant Proc. 2018;50: 2575–2578.
206. Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345: 747–755.
207. Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89: 1462–1465.
208. Velay A, Solis M, Benotmane I, Gantner P, Soulier E, Moulin B, et al. Intravenous Immunoglobulin Administration Significantly Increases BKPyV Genotype-Specific Neutralizing Antibody Titers in Kidney Transplant Recipients. Antimicrob Agents Chemother. 2019;63. doi:10.1128/AAC.00393-19
209. Vu D, Shah T, Ansari J, Naraghi R, Min D. Efficacy of intravenous immunoglobulin in the treatment of persistent BK viremia and BK virus nephropathy in renal transplant recipients. Transplant Proc. 2015;47: 394–398.
210. Sener A, House AA, Jevnikar AM, Boudville N, McAlister VC, Muirhead N, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81: 117–120.
211. Sharma AP, Moussa M, Casier S, Rehman F, Filler G, Grimmer J. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr Transplant. 2009;13: 123–129.
212. Benotmane I, Solis M, Velay A, Cognard N, Olagne J, Gautier Vargas G, et al. Intravenous immunoglobulin as a preventive strategy against BK virus viremia and BKV-associated nephropathy in kidney transplant recipients-Results from a proof-of-concept study. Am J Transplant. 2021;21: 329–337.
213. Abend JR, Sathe A, Wrobel MB, Knapp M, Xu L, Zhao L, et al. Nonclinical and clinical characterization of MAU868, a novel human-derived monoclonal neutralizing antibody targeting BK polyomavirus VP1. Am J Transplant. 2024;24: 1994–2006.
214. Jahan S, Scuderi C, Francis L, Neller MA, Rehan S, Crooks P, et al. T-cell adoptive immunotherapy for BK nephropathy in renal transplantation. Transpl Infect Dis. 2020;22: e13399.
215. Pfeiffer T, Tzannou I, Wu M, Ramos C, Sasa G, Martinez C, et al. Posoleucel, an Allogeneic, Off-the-Shelf Multivirus-Specific T-Cell Therapy, for the Treatment of Refractory Viral Infections in the Post-HCT Setting. Clin Cancer Res. 2023;29: 324–330.
216. AlloVir Provides Updates on Phase 3 Clinical Development Program for Posoleucel, an Allogeneic Virus-Specific T Cell Therapy. In: AlloVir [Internet]. 22 Dec 2023 [cited 22 Mar 2024]. Available: https://ir.allovir.com/news-releases/news-release-details/allovir-provides-updates-phase-3-clinical-development-program
217. Chandraker A, Regmi A, Gohh R, Sharma A, Woodle ES, Ansari MJ, et al. Posoleucel in Kidney Transplant Recipients with BK Viremia: Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. J Am Soc Nephrol. 2024. doi:10.1681/ASN.0000000000000329
218. Arora S, Setia R. Extracorporeal photopheresis: Review of technical aspects. Asian J Transfus Sci. 2017;11: 81–86.
219. Von Tokarski F, Parquin F, Roux A, Hayem V, Kerdiles T, Rabant M, et al. Successful prevention of BK-polyomavirus nephropathy using extracorporeal photopheresis for immunosuppression minimisation following severe BK polyomavirus replication after kidney transplantation in a double lung transplant recipient, a case report. BMC Nephrol. 2024;25: 363.
220. Xipell M, Molina-Andújar A, Cid J, Piñeiro GJ, Montagud-Marrahi E, Cofan F, et al. Immunogenic and immunotolerogenic effects of extracorporeal photopheresis in high immunological risk kidney recipients. A single center case series. J Clin Apher. 2022;37: 197–205.
221. Yuan X, Chen C, Zheng Y, Wang C. Conversion From Mycophenolates to Mizoribine Is Associated With Lower BK Virus Load in Kidney Transplant Recipients: A Prospective Study. Transplant Proc. 2018;50: 3356–3360.
222. Funahashi Y, Hattori R, Kinukawa T, Kimura H, Nishiyama Y, Gotoh M. Conversion from mycophenolate mofetil to mizoribine for patients with positive polyomavirus type BK in urine. Transplant Proc. 2008;40: 2268–2270.
223. Li P, Cheng D, Wen J, Ni X, Xie K, Li X, et al. Conversion from mycophenolate mofetil to mizoribine in the early stages of BK polyomavirus infection could improve kidney allograft prognosis: a single-center study from China. BMC Nephrol. 2021;22: 328.
224. Ishikawa H. Mizoribine and mycophenolate mofetil. Curr Med Chem. 1999;6: 575–597.
225. Acott PD, O’Regan PA, Lee SH, Crocker JF. Utilization of vero cells for primary and chronic BK virus infection. Transplant Proc. 2006;38: 3502–3505.
226. Panou M-M, Antoni M, Morgan EL, Loundras E-A, Wasson CW, Welberry-Smith M, et al. Glibenclamide inhibits BK polyomavirus infection in kidney cells through CFTR blockade. Antiviral Res. 2020;178: 104778.
227. Lorentzen EM, Henriksen S, Rinaldo CH. Modelling BK Polyomavirus dissemination and cytopathology using polarized human renal tubule epithelial cells. PLoS Pathog. 2023;19: e1011622.
228. Taguchi A, Nishinakamura R. Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell. 2017;21: 730–746.e6.
229. Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, Jansen J, et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol. 2019;37: 303–313.
5. Management of Treatment Failure
Treatment failure in the management of BKVAN is defined as persistence of BKPyV graft infection (often monitored using DNAaemia as a proxy) and consequential progressive irreversible graft deterioration leading to kidney transplant graft function loss.
Before diagnosing a patient with BKVAN treatment failure, the following are recommended:
- Perform a kidney transplant biopsy. This ensures clear diagnosis, and the exclusion of other pathology. It also allows prognostication and informs ongoing immunosuppression management [1D]
- Ensure the patient is on the minimised immunosuppression [1D], taking account of the need to balance this against the risk of:
- rejection (both acute and chronic)
- sensitisation (especially for young patients and those needing subsequent grafts)
- Optimisation of treatment through a multidisciplinary approach with transplant physicians, virologists and specialist transplant pharmacists; for instance in monitoring drug concentrations. [1C]
With current clinical practice in the UK, graft loss due to BKPyV infections is an uncommon outcome. Persistent DNAaemia (without evidence of ongoing graft deterioration or histological demonstration of BKVAN) may have implications for prognosis but this has yet to be clinically defined. Particular attention should be given to paediatric cases of BKVAN where graft loss appears to be more common and paediatric patients are more likely to need re-transplantation.
5.1 In determining the reasons for treatment failure in BKVAN, it is essential to exclude any other cause of transplant failure. Ideally, all graft failures should be evaluated by histology of renal biopsy for the presence of BKPyV, to determine the extent of disease and to distinguish between infection and rejection. [1B]
5.1.1 In cases where a biopsy is SV40 large T antigen positive but the patient was negative for BKPyV DNAaemia, JCPyV DNAaemia testing is clinically appropriate.
5.1.2 Biopsies should be examined for concomitant rejection.
5.2 Previous transplant loss due to BKPyV should not preclude patients from re-transplantation as re-transplantation is often successful following BKVAN in an earlier transplant. Re-transplantation should not be excluded, particularly in the younger patient. [1C]
5.2.1 Ideally, clearance of BKPyV infection / DNAemia should be achieved before re-transplantation. This is often observed when immunosuppression is reduced following graft loss.
5.2.2. Increasing use of ongoing immunosuppression after graft failure (in line with UK and international guidelines) means some patients requiring re-transplantation may not reduce immunosuppression to the level required to see complete clearance of BKPyV DNAaemia. This should not be a contraindication to re-listing.
5.2.3 Some patients may be established on dialysis and still have equivocal BKPyV DNAaemia despite immunosuppression withdrawal. These patients should be monitored for BKPyV DNAaemia every 1-3 months.
5.2.4 Counselling of re-transplanted patients following loss of graft to BKVAN is important to manage the risk of losing the new graft to BKPyV.
5.3 Nephrectomy motivated by clearance of BKPyV infection is controversial and does not have the evidence base required to make recommendations.
Research recommendations:
- Genotyping in cases of graft failure to identify whether certain BKPyV variants are more likely to be associated with graft loss.
- Potential role of new, more targeted therapies as they become available.
- Larger multi-centre studies describing the natural history of retransplantation following graft loss due to BKVAN.
Audit Measures
- Proportion of patients losing their graft due to irreversible BKVAN and requiring renal replacement therapy.
- Timing of re-transplant relative to diagnosis of resistant BKVAN.
- BKPyV DNAaemia IU/mL at retransplant.
- Proportion of recurrence of BKVAN in those re-transplanted
- Graft survival post retransplant in those with low level BKPyV DNAaemia vs those transplanted with undetectable DNAaemia.
Rationale
Factors that increase the tendency to develop BKVAN are also likely to increase the likelihood of treatment failure. They are considered as non-modifiable such as older age (viruria in the general population increases linearly with age [21]), male sex, caucasian, diabetes mellitus, donor seropositivity and recipient seronegativity, poor cellular immune response and recent transplant year [230,231].
Viral Load & Renal Allograft Nephrectomy
One retrospective case series assessed BKPyV DNAaemia changes in response to allograft nephrectomy in 3 cases [232]. One patient continued immunosuppression for a pancreas graft and two with continued immunosuppression to limit allosensitisation before repeat transplant [232]. Despite the ongoing immunosuppression, BKPyV DNAaemia load decreased following nephrectomy in all 3 cases [232].
Nephrectomy & Sensitisation
Successful outcomes for retransplantations, following any cause of graft failure, have been reported with and without primary allograft nephrectomy; as covered in detail by a previous BTS Guideline ‘Management of the Failing Kidney Transplant’ [233].
A systematic review of 15 studies revealed no advantage in graft or patient survival with transplant nephrectomy prior to re-transplant, following any cause of graft failure [234]. There is an increased risk of sensitisation after allograft nephrectomy, warranting caution in highly sensitised patients [234]. Other reviews and single centre experiences agree with increased risk of sensitisation [235–239]. There has been limited research into the outcomes for retransplantation in patients with BKPyV DNAaemia. Certainly, there have been successful retransplantations with ongoing BKPyV DNAaemia and without nephrectomy reported [240,241], although data regarding longer term outcomes is lacking.
A lack of quality evidence makes comparison between intra- and extra-capsular allograft nephrectomy difficult, but no significant differences are observed in nephrectomies for other conditions (covered in [233]).
Re-transplantation and Timing
A history of BKVAN should not be considered a contraindication for re-transplantation (and this is particularly true in the younger patient). There are numerous case reports of successful retransplantation following graft loss attributed to BKVAN, with the largest dataset coming from a retrospective review of OPTN data [242]. Between 2004 and 2008, of 127 patients were re-transplanted following graft loss due to BKVAN, one and three year allograft survival were 98.5% and 93.7%, respectively [242]. A more recent review of OPTN data between 2005-2016 reported death-censored 5-year graft survival post-BKVAN of 90.6%, which was greater than allograft survival for those retransplanted following graft failure for other reasons (83.9%) [243].
There is insufficient evidence to define a recommendation on the timing and precise BKPyV DNAaemia titres that enable a re-transplantation free from viral reactivation. One retrospective study of 31 patients in 6 US centres suggested that documented clearance of BKPyV DNAaemia prior to retransplantation was associated with the absence of BKPyV replication after re-transplantation [244]. All five patients with BKPyV DNAaemia before re-transplant experienced BKPyV replication after; leading the authors to conclude retransplant was preferable post-BKPyV clearance [244].
However, patients with active BKVAN and BKPyV DNAaemia might not be excluded from immediate re-transplantation because there is the opportunity to clear the infection post-retransplantation. One study reports two successful preemptive re-transplantations (with simultaneous allograft nephrectomy) in patients with active BKPyV DNAaemia and BKVAN [245]. At 12- and 21-month follow-up, both patients had stable allograft function and no evidence of active BKPyV DNAaemia [245]. The authors conclude that preemptive retransplantation can be considered in patients with allografts failing due to BKVAN [245].
Patients diagnosed with a failing graft as a result of BKVAN should be established on the unit pathway for failing allografts similar to failing grafts due to other causes (see BTS Guideline ‘Management of the Failing Kidney Transplant’ [233]).
References
21. Zhong S, Zheng H-Y, Suzuki M, Chen Q, Ikegaya H, Aoki N, et al. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J Clin Microbiol. 2007;45: 193–198.
230. Lorant C, Westman G, Bergqvist A, von Zur-Mühlen B, Eriksson B-M. Risk factors for developing BK virus-associated nephropathy: A single-center retrospective cohort study of kidney transplant recipients. Ann Transplant. 2022;27: e934738.
231. Demey B, Tinez C, François C, Helle F, Choukroun G, Duverlie G, et al. Risk factors for BK virus viremia and nephropathy after kidney transplantation: A systematic review. J Clin Virol. 2018;109: 6–12.
232. Funk GA, Steiger J, Hirsch HH. Rapid Dynamics of Polyomavirus Type BK in Renal Transplant Recipients. J Infect Dis. 2006;193: 80–87.
233. UK GUIDELINE FOR THE MANAGEMENT OF THE PATIENT WITH A FAILING KIDNEY TRANSPLANT. In: British Transplantation Society [Internet]. 16 Jun 2023 [cited 3 Mar 2024]. Available: https://bts.org.uk/uk-guideline-for-the-management-of-the-patient-with-a-failing-kidney-transplant/
234. Vlachopanos G, El Kossi M, Aziz D, Halawa A. Association of Nephrectomy of the Failed Renal Allograft With Outcome of the Future Transplant: A Systematic Review. Exp Clin Transplant. 2022;20: 1–11.
235. Lucisano G, Brookes P, Santos-Nunez E, Firmin N, Gunby N, Hassan S, et al. Allosensitization after transplant failure: the role of graft nephrectomy and immunosuppression – a retrospective study. Transpl Int. 2019;32: 949–959.
236. Muramatsu M, Hyodo Y, Sheaff M, Gupta A, Ashman N, Aikawa A, et al. Impact of allograft nephrectomy on second renal transplant outcome. Exp Clin Transplant. 2018;16: 259–265.
237. Singh P, Filippone EJ, Colombe BW, Shah AP, Zhan T, Harach M, et al. Sensitization trends after renal allograft failure: the role of DQ eplet mismatches in becoming highly sensitized. Clin Transplant. 2016;30: 71–80.
238. Knight MG, Tiong HY, Li J, Pidwell D, Goldfarb D. Transplant Nephrectomy After Allograft Failure Is Associated With Allosensitization. Urology. 2011;78: 314–318.
239. Douzdjian V, Rice JC, Carson RW, Gugliuzza KK, Fish JC. Renal retransplants: effect of primary allograft nephrectomy on early function, acute rejection and outcome. Clin Transplant. 1996;10: 203–208.
240. Huang J, Danovitch G, Pham P-T, Bunnapradist S, Huang E. Kidney retransplantation for BK virus nephropathy with active viremia without allograft nephrectomy. J Nephrol. 2015;28: 773–777.
241. Ginevri F, Pastorino N, de Santis R, Fontana I, Sementa A, Losurdo G, et al. Retransplantation after kidney graft loss due to polyoma BK virus nephropathy: successful outcome without original allograft nephrectomy. Am J Kidney Dis. 2003;42: 821–825.
242. Dharnidharka VR, Cherikh WS, Neff R, Cheng Y, Abbott KC. Retransplantation after BK virus nephropathy in prior kidney transplant: an OPTN database analysis. Am J Transplant. 2010;10: 1312–1315.
243. Leeaphorn N, Thongprayoon C, Chon WJ, Cummings LS, Mao MA, Cheungpasitporn W. Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Am J Transplant. 2020;20: 1334–1340.
244. Geetha D, Sozio SM, Ghanta M, Josephson M, Shapiro R, Dadhania D, et al. Results of repeat renal transplantation after graft loss from BK virus nephropathy. Transplantation. 2011;92: 781–786.
245. Womer KL, Meier-Kriesche H-U, Patton PR, Dibadj K, Bucci CM, Foley D, et al. Preemptive Retransplantation for BK Virus Nephropathy: Successful Outcome Despite Active Viremia. Am J Transplant. 2006;6: 209–213.
6. Information, education and support
6.1 Reliable information should be provided to patients at the point of diagnosis.
6.2 Use the BTS patient information leaflet (PIL), co-developed with patients to give balanced information at initial diagnosis of BKPyV infection in transplant recipients.
6.3 Offer balanced and accurate information about:
- The risks of BKPyV infection and disease
- The nature of immunosuppression changes
- Monitoring approach
6.4 Ensure that healthcare professionals offering information have specialist knowledge about BKPyV infection and disease and their treatment, and the skills to support shared decision-making (for example, presenting information in a form suitable for developmental stage).
Research recommendations:
- Development of BKPyV patient information tailored to the needs of children and young people.
Rationale
As part of the development of these guidelines, a sub-group of the authors co-developed an information leaflet with patients to address the abundance of unreliable information on the internet which is a significant cause of stress for transplant-recipients receiving positive BKPyV DNAaemia results. The patient information is now available via the National Kidney Federation website (https://www.kidney.org.uk/bk-virus) and a version was published in the Journal of Kidney Care [246].
There was very little reliable patient-oriented information available for those diagnosed with a BKPyV infection on the internet. The best resources, in addition to the patient information noted above, have been developed by charities and include:
- BK virus-associated nephropathy – Kidney Research UK [247]
- BK Virus: What Transplant Patients Need to Know – National Kidney Foundation [248]
None of the available resources (that we could find) have been written explicitly for children and young people.
References
246. Baker SC, Al-Talib M, Welberry Smith M. BK polyomavirus. J Kidney Care. 2024;9: 236–238.
247. BK virus-associated nephropathy. In: Kidney Research UK [Internet]. [cited 28 Mar 2024]. Available: https://www.kidneyresearchuk.org/conditions-symptoms/bk-virus/
248. BK Virus: What Transplant Patients Need to Know. In: National Kidney Foundation [Internet]. [cited 28 Mar 2024].
Available: https://www.kidney.org/atoz/content/bk-virus-what-transplant-patients-need-know