UK GUIDELINE ON PREVENTION AND MANAGEMENT OF CYTOMEGALOVIRUS (CMV) INFECTION AND DISEASE FOLLOWING SOLID ORGAN TRANSPLANTATION
Table of Contents
EXECUTIVE SUMMARY OF RECOMMENDATIONS
EXECUTIVE SUMMARY OF RESEARCH RECOMMENDATIONS
EXECUTIVE SUMMARY OF AUDIT MEASURES
Background to Cytomegalovirus (CMV)
Process of Writing and Methodology
1 Preventing CMV Infection and Disease
Determining CMV status in donor and recipient
3 Monitoring CMV Infection and Disease
4 Treating CMV Infection and Disease
6 Immunosuppression dose reduction
Executive Summary of Recommendations
1 Preventing CMV Infection and Disease
Determining CMV status in donor and recipient
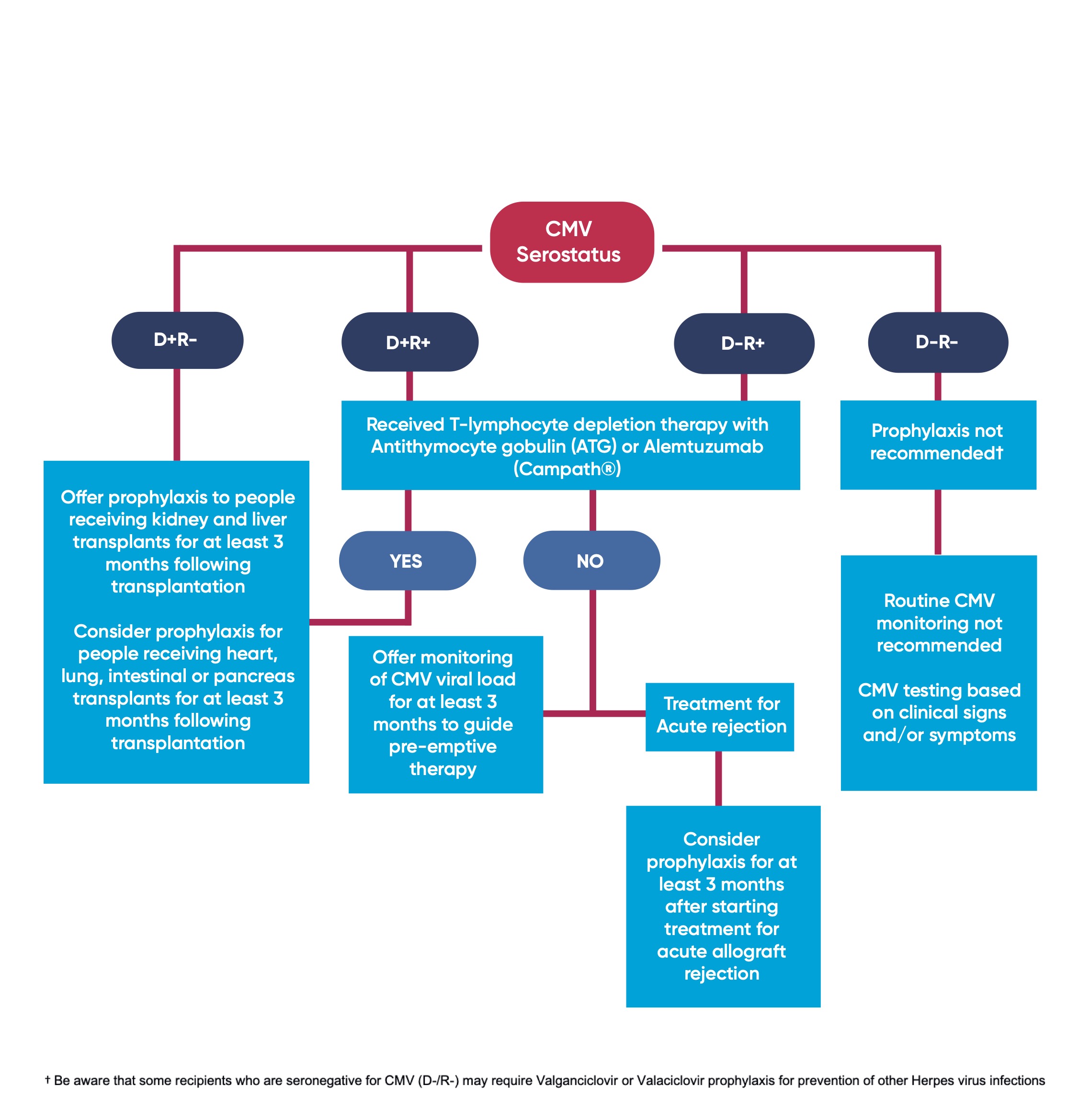
1.1 All organ donors and recipients should be screened for CMV (IgG antibody) serostatus prior to, or at the time of transplantation [1A]
1.2 If donor CMV serostatus is unknown and recipient is seronegative or unknown for CMV, this should be characterised as donor seropositive, recipient seronegative for CMV (D+/R-). [1D]
CMV prophylaxis
For adults, children and young people receiving a solid organ transplant:
1.3 Offer Valganciclovir prophylaxis to people receiving kidney and liver transplants for at least 3 months following transplantation if either:
- The recipient is seronegative for CMV and receives an allograft from a CMV seropositive donor (D+/R-) [1A] OR
- The recipient has received T-lymphocyte depletion therapy with Antithymocyte gobulin (ATG) or Alemtuzumab (Campath®), where donor: recipient serostatus is D+/R+ or D-/R+ [1A]
1.4 Consider Valganciclovir prophylaxis for people receiving heart, lung, intestinal or pancreas transplants for at least 3 months following transplantation if either:
- The recipient is seronegative for CMV and receives an allograft from a CMV seropositive donor (D+/R-) [1C] OR
- The recipient has received T-lymphocyte depletion therapy with Antithymocyte gobulin (ATG) or Alemtuzumab (Campath®), where donor: recipient serostatus is D+/R+ or D-/R+ [1C]
1.5 Do not routinely offer Valganciclovir prophylaxis if donor and recipient are seronegative for CMV (D-/R-) [1D]
1.5.1 Be aware that some recipients who are seronegative for CMV (D-/R-) may require Valganciclovir or Valaciclovir prophylaxis for prevention of other Herpes virus infections, e.g; recipients who are seronegative for Herpes Simplex Virus (HSV) receiving an organ from HSV seropositive donors [2D].
1.6 Consider Valganciclovir prophylaxis for at least 3 months after starting treatment for acute allograft rejection if either donor or recipient are CMV positive (D+/R-, D+/R+ or D-/R+) [2C].
1.7 Adjust the dose of Valganciclovir if creatinine clearance is less than 60ml/minute [1D]
1.8 For children and young people under 18 years, adjust the dose of Valganciclovir according to body surface area, up to a maximum dose of 900mg once daily [1D]
1.9 Monitor Full Blood Count at least every 2 weeks whilst on Valganciclovir [1A]
1.10 Consider switching to CMV monitoring and pre-emptive therapy if people develop side effects e.g.; neutropenia, on Valganciclovir [1C]
2 Laboratory Testing for CMV
2.1 Clinical Laboratories should use Quantitative Nucleic Acid Testing (QNAT) of whole blood or plasma to quantify CMV viral load [1A]
- Transplant centres should adopt a consistent approach to use either whole blood or plasma for quantification of CMV viral load [1C]
- CMV QNAT assays should be calibrated using the WHO International Reference Standard [1C]
- CMV viral load should be reported as IU/mL, where possible, or, if reported as copies / mL, a conversion factor should be used [1C]
2.2 Transplant centres should determine local thresholds of viral load that portend CMV infection or disease requiring treatment, depending on the CMV QNAT assay they use and the population at risk [1C]
2.3 QNAT thresholds should not be used in isolation to determine commencement or cessation of treatment for individuals with CMV disease, but should be considered in the context of clinical, laboratory or histological evidence of CMV disease [1C]
3 Monitoring for CMV Infection and Disease
3.1 Offer monitoring of CMV viral load to guide pre-emptive therapy to adults, children and young people receiving a solid organ transplant if:
- They are CMV seropositive (D+/R+, D-/R+) [1B] AND
- They have not received T-lymphocyte depletion therapy [1B] AND
- They are not receiving valganciclovir prophylaxis [1B] (see section 1)
3.2 Where CMV viral load monitoring is offered:
- Consider testing with a frequency of at least monthly [1D]
- Consider testing for at least 3 months following transplantation [1D]
4 Treating CMV Infection and Disease
For adults, children and young people who develop CMV infection or disease following solid organ transplantation:
4.1 Offer treatment with oral Valganciclovir for a duration of at least 2 weeks [1A]
4.2 Adjust the dose of Valganciclovir according to licensed dosing recommendations if creatinine clearance is less than 60ml/minute [1D]
4.3 Be aware of the potential development of ganciclovir resistance (see section 5 for management)
4.4 Assess CMV viral load after 2 weeks of treatment and repeat at a minimum interval of 7 days [1D] AND
- Consider stopping treatment for CMV disease after resolution of symptoms AND two consecutive, CMV viral load tests that confirm that CMV is not detected (below the local laboratory threshold for detection) [2D].
5 Ganciclovir resistance
5.1 Be aware that the following people are at increased risk of Ganciclovir resistance:
- Those who have received prolonged courses, or sub-therapeutic doses of antiviral medications [1A]
- CMV seronegative recipients receiving a solid organ transplant from seropositive donors (D+/R-) [1C]
- Those who have received intensive immunosuppression (e.g; with T-cell depletion therapy, following episodes of acute allograft rejection) [1B]
- Recipients of lung transplants [2C]
5.2 Consider resistance to Ganciclovir if:
- There is a persistent or increasing viral load or symptomatic disease after a normally effective dosage and duration (e.g.; 2-4 weeks) of Ganciclovir or Valganciclovir [1A]
5.3 In people with suspected resistance to Ganciclovir:
- Confirm that dosing is adequate
- Consider adherence to treatment plan and absorption
- Offer testing for Human CMV (HCMV) antiviral resistance
- UL97 and UL54 gene mutations [1A]
- Use either whole blood or plasma [1A]
- Consider switching Valganciclovir (or Ganciclovir) to oral Maribavir for 8 weeks (not licensed in children) [2D]
5.4 In people with evidence of ganciclovir resistance:
- Seek specialist virology advice [1D]
- Stop Valganciclovir (or Ganciclovir) [1A]
- Offer oral Maribavir for 8 weeks (not licensed in children) [1B] OR
- Offer Intravenous Foscarnet for at least 3 weeks [1B]
- Seek specialist virology advice before commencing treatment with Foscarnet [1D]
6 Immunosuppression dose reduction
For adults, children and young people who develop CMV infection or disease (with or without leukopenia) following solid organ transplantation:
6.1 Consider a dose reduction of either calcineurin inhibitor or mycophenolate mofetil / azathioprine [1C]
- Consider preferentially reducing or stopping Mycophenolate Mofetil (MMF) or azathioprine if there is evidence of leukopenia [2C]
6.2 Discuss with patients, and, where appropriate, parents or carers, the risk of acute rejection with immunosuppression dose reduction [1D]
6.3 Review the dosing of immunosuppression following resolution of CMV infection or disease [1D]
7 Information, education and support
7.1 Offer balanced and accurate information to children, adults and young people undergoing solid organ transplantation about:
- The risks of CMV infection and disease according to donor / recipient CMV serostatus and nature of immunosuppression planned or received
- Monitoring and treatment options, including prophylaxis
7.2 Ensure that healthcare professionals offering information have specialist knowledge about CMV infection and disease and their treatment, and the skills to support shared decision-making (for example, presenting information in a form suitable for developmental stage)
Executive Summary of Research Recommendations
In adults, children and young people receiving solid organ transplants:
- What is the clinical and cost-effectiveness of anti-CMV prophylaxis vs active surveillance (monitoring followed by pre-emptive therapy), for people receiving heart, lung, liver, intestinal or pancreas transplants for at least 3 months following transplantation?
- What is the clinical and cost-effectiveness of anti CMV prophylaxis, vs active surveillance (monitoring followed by pre emptive therapy), following treatment of CMV infection or disease?
- What is the most clinical and cost-effective duration and frequency of CMV viral load monitoring?
- What is the clinical and cost-effectiveness of CMV viral load monitoring following cessation of anti CMV prophylaxis?
- What is the clinical and cost-effectiveness of CMV viral load monitoring following completion of treatment of CMV infection or disease?
- What is the clinical and cost-effectiveness of CMV immune monitoring?
- What is the clinical and cost-effectiveness of using CMV viral load (QNAT) testing to guide cessation of treatment of CMV infection and disease?
- In adults, children and young people who develop CMV infection or disease (with or without leukopenia) following solid organ transplantation, is reduction of Calcineurin Inhibitors (CNIs) or proliferation inhibitors associated with better outcomes?
- What is the clinical and cost-effectivenessof CMV specific immunoglobulins and CMV specific cytotoxic T cells in the treatment of severe, resistant or refractory CMV disease?
In children and young people receiving solid organ transplants:
- What is the most clinical and cost-effective calculation for dosing Valganciclovir according to body surface area?
Executive Summary of Audit Measures
- Proportion of adults, children and young people receiving solid organ transplants who are offered Valganciclovir prophylaxis according to donor-recipient CMV profiles and use of T-cell depletion therapy as set out in recommendation 1.3
- Proportion of Clinical Laboratories using Quantitative Nucleic Acid Testing (QNAT) of whole blood or plasma to quantify CMV viral load
- Proportion of CMV QNAT assays calibrated using the WHO International Reference Standard.
- Proportion of adults, children and young people characterised in recommendation 3.1 who are offered monthly CMV viral load monitoring for 3 months following solid organ transplantation
- Proportion of adults, children and young people who are offered Valganciclovir to treat CMV infection and disease following solid organ transplantation
- Proportion of adults, children and young people with Ganciclovir resistance tested for UL97 and UL54 mutations.
- Proportion of adults, children and young people who develop CMV infection or disease (with or without leukopenia) following solid organ transplantation, and who reduce or stop Calcineurin Inhibitors (CNIs) and / or proliferation inhibitors
INTRODUCTION
Background to Cytomegalovirus (CMV)
Biology of CMV in Humans
CMV is one of the herpes group of viruses, which are widely distributed among mammals. The various strains of CMV are species specific and primary infection results in the most severe disease when the host is T-cell immunocompromised. After primary CMV infection, the viral genome enters monocytes and other bone marrow progenitor cells and enters a latent state.
The prevalence of antibody indicating previous infection increases with age in all human populations that have been studied. The prevalence of past exposure to CMV, as indicated by positive IgG antibody (serostatus), varies markedly throughout the world [1]. In developed countries, the percentage of the population who are seropositive has been reported to increase roughly linearly with age; approximately 40% at age 20 and 80% at age 60 [2]. Transmission occurs from direct person-to-person contact through exposure to saliva, urine, breast milk or genital secretions.
CMV in Solid Organ Transplant Recipients
Primary infection with CMV typically occurs approximately four to six weeks post-transplant in a seronegative individual who receives a seropositive organ. Symptoms due to primary disease may occur as early as 20 days and are rare more than 50 days post-transplantation provided the patient has not received antiviral drugs [3]. Symptoms may be non-specific, such as fever, night sweats, fatigue and myalgia. Retinitis can be pathognomonic, but is rarely seen in the transplant population. Gastrointestinal disease presenting with diarrhoea, abdominal pain and nausea is common and respiratory distress may be an indication of pulmonary involvement. Routine blood tests may detect bone marrow suppression, especially neutropenia, as well as biochemical hepatitis, as evidenced by fluctuations in ALT and AST.
After a primary infection an individual would be expected to mount IgM and later an IgG immunoglobulin response against CMV. In immunocompetent individuals, the presence of IgM and/or low avidity IgG antibodies is a hallmark of primary infection. In the immunosuppressed, however, the antibody rise may be delayed or absent. The presence of IgG is used to determine prior exposure of both donors and recipients to CMV infection, most commonly at the time of transplant wait listing, and of donors at the time of organ retrieval. Donor: recipient CMV serostatus is characterised as:
- Both donor and recipient are IgG antibody seropositive:
- Donor (D) positive (+); Recipient (R) positive (+) (D+/R+)
- Both donor and recipient are IgG antibody seronegative:
- Donor (D) negative (-); Recipient (R) negative (-) (D-/R-)
- Donor is IgG antibody seropositive and recipient is IgG antibody seronegative:
- Donor (D) positive (+); Recipient (R) negative (-) (D+/R-)
- Donor is IgG antibody seronegative and recipient is IgG antibody seropositive:
- Donor (D) negative (-); Recipient (R) positive (+) (D-/R+)
Tests based on the polymerase chain reaction (PCR) carried out on plasma, whole blood or leukocytes have become the gold standard means of detecting active CMV replication in many laboratories in the UK and beyond. CMV load, used as a surrogate marker of CMV replication, has been shown in many studies to be a dominant risk factor for CMV disease. Approaches that maintain viral replication at very low levels in the early post-transplant period (prevention or prophylaxis) or which rapidly reduce viral load when a certain level has been reached (pre-emptive therapy) have become the principal methods of controlling CMV disease. Uncertainty as to whether it is best to test plasma or whole blood and the lack of an international reference standard has made the comparison of cut-offs for the initiation of antiviral therapy difficult to compare between laboratories and problematic when patients migrate between different care centres.
On occasion it is necessary to prove CMV organ specific dysfunction by obtaining a biopsy. Liver (native and allograft), gastrointestinal, bone marrow, lung and renal allograft biopsies can be diagnostic.
The frequency of CMV disease in solid organ transplant (SOT) recipients varies markedly depending on the definition of CMV disease that is used and the intensity of immunosuppression. In the ‘pre-prophylaxis era’, approximately 8% of kidney, 29% of liver, 25% of heart and 39% of lung transplants could be expected to experience symptomatic CMV infection [4]. More recently in kidney and liver transplant recipients who received prophylaxis, the 1-year incidence of CMV disease was reported to be 19.2% and 31.3%, respectively in D+ / R- group and 2.5% and 3.2%, respectively, in D- / R- group [5]. In heart transplant recipients who received antiviral prophylaxis in the first month after transplant followed by pre-emptive therapy, the cumulative incidence of CMV infection and disease during the first year was reported to be 47% and 7.5%, respectively [6]. The incidence of CMV disease among lung transplant recipients who received antiviral prophylaxis for 6 to 12 months was 14.9%, with a higher incidence (26.6%) in D+ /R- group [7]. Most cases of CMV disease in SOT recipients who received antiviral prophylaxis occur after cessation of antiviral drug administration, hence the term ‘late-onset CMV disease’, and they occur predominantly in CMV mismatch (D+ / R-) recipients. Late-onset CMV disease remains associated with allograft failure and mortality [8,9].
There are conflicting reports about the impact of CMV on patient and graft outcomes following solid organ transplantation. Early studies (prior to the era of anti CMV prophylaxis) reported fatality rates of up to 30% in seronegative recipients of seropositive kidneys who received Anti-Lymphocyte Globulin [10].
CMV infection can be coincident with acute allograft rejection [11]. Early studies reported a 50% reduction in biopsy-proven acute graft rejection by anti-CMV prophylaxis in D+/R- kidney transplant recipients [12].
In a single centre study of 1,339 kidney transplant recipients, multivariate analysis showed that CMV disease appeared to influence long term graft survival but only when coupled with the occurrence of acute rejection [13]. In a liver transplant population of 33 patients receiving 57 transplants, persistent CMV infection, defined by serial PCR measurements was significantly associated with graft loss through chronic rejection. However, there was no significant correlation between primary infection or symptomatic disease and chronic rejection, possibly as a consequence of the small sample size [14]. A more recent publication examined graft outcome in 10,190 kidney transplants performed in adults, children and young people in the UK between 2000-7. After adjustment for donor age, this showed no significant effect of donor or recipient CMV status on either allograft or patient survival at three years post-transplant [15].
Preventing CMV disease in people with solid organ transplants
Previous attempts to minimise the risk of CMV disease have included avoiding transplanting a seropositive organ into a seronegative recipient; not a viable strategy in the context of the life-sustaining nature of solid organ transplantation. Another theoretically attractive option for prevention would be vaccination against CMV. However, the heterogeneity of strains has limited the yield from vaccination as a prophylactic strategy.
Passive immunoprophylaxis has been explored in solid organ transplantation in randomised trials since the 1980s [16-19]. An early placebo-controlled study in patients receiving a liver transplant, reported significant overall protection from severe disease by hyperimmune globulin, but limited or no protection in the D+/R- sub-group. Intravenous treatment is generally less convenient for the patient and health care provider, and caries the theoretical risk of transmitting blood-borne viruses (and vCJD in the UK). However, it does have the advantage of allowing compliance to be documented and on occasions this may have significant advantages. The limited availability of this product restricts the usefulness of this approach
Universal anti-CMV Prophylaxis and pre-emptive anti-CMV therapy are in common use to minimise the impact of CMV infection or reactivation. In some units, both approaches are employed, depending upon the donor/recipient CMV status, organs transplanted, and intensity of immunosuppression. Aciclovir, Valaciclovir, Ganciclovir and Valganciclovir have all been used for prophylaxis. Newer agents such as Letermovir and Maribavir may have a role in prevention of CMV disease. In pre-emptive prophylactic strategies, transplant recipients undergo regular surveillance and are treated when judged to be at high risk of developing CMV disease. The absolute levels of viraemia that are recommended as a threshold to start pre-emptive therapy will depend upon the assay used. Units should establish the clinical significance of their local assay. The availability of a universal standard for molecular assays for CMV would facilitate cross-comparisons of viral load measurements from different laboratories and facilitate future multicentre trials without the explicit need for centralised testing.
Treatment of CMV Disease
Early studies emphasised the need to reduce immunosuppression as well as giving specific antiviral therapy [20, 21]. Intravenous Ganciclovir has had a major impact on the mortality and morbidity of CMV disease [21-23]. High doses of intravenous Hyperimmune globulin (0.5 g/kg body weight) have been used in conjunction with Ganciclovir for the treatment of pneumonitis [24].
There is a significant risk of relapse following successful treatment of CMV disease, with recurrent CMV disease reported in various organ recipients [25-29]. Secondary prophylaxis after treatment of disease has been assessed, although evidence in support of this strategy is limited [30].
Antiviral drug Resistance
In clinical practice, antiviral drug resistance is manifest by either progressive disease despite full dose anti-viral therapy, or a static or increasing viral load after drug treatment. Importantly, viral load is not a reliable indicator of drug resistance in the first week of treatment, since the natural kinetics of CMV replication prior to antiviral treatment means that patients with a rapidly increasing viral load are more likely to show a transient increase in viral load in the first 5-7 days after therapy, before a decline is observed [31].
Genotypic assays can relatively rapidly detect gene mutations that are associated with both high- and low-grade resistance to ganciclovir as well as mutations that confer various degrees of resistance to Foscarnet and Cidofovir. This is a rapidly evolving field with more laboratories having the capability to offer high throughput sequence-based analysis of UL97 and UL54 gene mutations. Close liaison between transplant staff and local virological expertise is necessary to exclude the other reasons for non-response, including adherence to treatment, prior to performing a relatively expensive drug resistance profile. Cidofovir is a potential second line therapy unless there is a UL54 genotype that confers both Ganciclovir and Cidofovir resistance [32]). It has significant side effects including nephrotoxicity. Foscarnet is reserved as second or even third line therapy partly because of significant risks of nephrotoxicity and electrolyte disturbances. The use of Maribavir for treating refractory or resistant CMV infection following solid organ transplantation has been assessed in phase 3 studies and reported to be superior to Ganciclovir, Cidofovir and Foscarnet [33]. Maribavir has Food and Drugs Administration (FDA) and Medicines and Healthcare products Regulatory Agency (MHRA) approval and is now recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of CMV infection and/or disease that is refractory (with or without resistance) to one or more prior therapies, including valganciclovir, ganciclovir, cidofovir and foscarnet in adults who have undergone a solid organ transplant [34].
The Need for The Guideline
As set out above, CMV is one of the most common infectious complications of solid organ transplantation and is reported to increase graft loss and patient mortality. Practice in the UK, however, varies across many areas of care, including indications for, and duration of anti-viral prophylaxis and monitoring of CMV. Uncertainty also exists with regard to CMV testing and the lack of an international reference standard has made the comparison of cut-offs for the initiation of antiviral therapy difficult to compare between laboratories and problematic when patients migrate between different care centres.
This guideline makes recommendations for the prevention and management of Cytomegalovirus (CMV) infection and disease and updates the previous version published in 2015. A full health economic assessment was not possible within existing resources.
Process of Writing and Methodology
The guideline was developed in accordance with The British Transplantation Society (BTS) Guideline Development Policy [35]. The BTS formed a guideline committee (GC) in June 2021. A draft scope was developed and sent to agreed stakeholders for consultation in September 2021. The guideline committee defined a series of key clinical questions relating to the defined scope, which were expressed in PICO (population, intervention, comparator, outcome) format. The Centre for Evidence in Transplantation (CET) was commissioned to undertake systematic literature searches for each key clinical question. Inclusion and exclusion criteria were defined from the clinical questions, and a search strategy was devised using both free text and keyword terms. Medline, EMBASE and the Transplant Library (all 1980 – 2021) databases were searched as well as websites of national associations in this field between October and December 2021. The clinical leads also hand searched reference lists of reviews and included papers.
Abstracts were screened for relevance by members of the CET, according to the pre-defined inclusion and exclusion criteria. Abstracts identified for review by reviewers were compared and any disputed abstracts were resolved by the guideline committee. The full papers were then assessed by the GC to further exclude any study that did not meet the following predefined criteria: Randomised controlled trials (RCT), non-randomised studies if adjusted for key confounders (age, health at baseline, co-morbidities).
Members of the CET and clinicians on the guideline committee critically appraised eligible papers using Critical Appraisal Skills Programme (CASP) tools. This is set of critical appraisal tools are designed to assess and interpret evidence, by systematically considering its validity, results and relevance within the field of study [36].
Contributing Authors
Dr Jan Dudley, Consultant Paediatric Nephrologist, Bristol Royal Hospital for Children (chair)
Dr Elham Asgari, Consultant Nephrologist, Guys and St Thomas NHS Foundation Trust
Dr Sai Bhagra, Consultant Cardiologist, Advanced Heart Failure and Cardiac Transplantation, Royal Papworth Hospital, Cambridge
Dr Angus Hann, Clinical Research Fellow, The Liver Unit, Queen Elizabeth Hospital, Birmingham (lead reviewer)
Dr Tanzina Haque, Consultant Virologist, Royal Free London NHS Foundation Trust
Mr John O’Callaghan, Transplant Surgeon, Centre for Evidence in Transplantation
Dr Rachel Hilton, Consultant Nephrologist, Guys and St Thomas NHS Foundation Trust
Mr Simon Knight, Senior Clinical Research Fellow and Honorary Consultant Transplant Surgeon, Nuffield Department of Surgical Sciences, Centre for Evidence in Transplantation, University of Oxford
Dr Farah E Latif, Nephrology Speciality Trainee and WCAT Clinical Lecturer, Nephrology & Transplant Directorate, University Hospital of Wales, Cardiff (lead reviewer)
Ms Sue Lyon, kidney transplant recipient, London
Mrs Shashi Matharu, kidney transplant recipient, West Bromwich
Dr Joanna Moore, Consultant Hepatologist and Honorary Senior Clinical Lecturer,
Leeds Liver Unit, St James’s University Hospital, Leeds
Professor David Mutimer, Consultant in Liver Medicine, The Liver Unit, Queen Elizabeth Hospital, Birmingham
Dr Jasvir Parmar, Lung Transplant Physician, Cardio-Thoracic Transplant Unit Royal Papworth Hospital in Cambridge
Dr Liset Pengel, Co-Director, Peter Morris Centre for Evidence in Transplantation
Nuffield Department of Surgical Sciences, University of Oxford, John Radcliffe Hospital Headington, Oxford
Dr Mysore Phanish, Consultant Nephrologist, Renal Unit, St Helier Hospital, Epsom and St Helier University Hospitals NHS trust, St Georges’ University of London
Ms Linda Ross, Clinical Pharmacist in Renal Transplantation and Urology · Guy’s and St Thomas’ NHS Foundation Trust
Dr Rishana Shuaib, Specialist Trainee, Nephrology, Imperial College Healthcare NHS Trust, London (lead reviewer)
Dr Matt Welberry Smith, Consultant Nephrologist, Leeds Teaching Hospital NHS Trust
Dr Ines Ushiro-Lumb, Lead Clinical Microbiologist for Organ and Tissue Donation and Transplantation, NHSBT
Conflicts of Interest
None. All authors made declarations of interest at every committee meeting and in line with the BTS Guideline Development policy. Further details can be obtained on request.
Grading of Recommendations
These guidelines represent consensus opinion from experts in the field of transplantation in the United Kingdom. They represent a snapshot of evidence available at the time of writing. It is recognised that recommendations are made even when the evidence is weak. It is felt that this is helpful to clinicians in daily practice.
In these guidelines the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system has been used to rate the strength of evidence and the strength of recommendations [37]. The approach used in producing the present guidelines is consistent with that adopted by Kidney Disease Improving Global Outcomes (KDIGO) [38, 39]. Explicit recommendations are made on the basis of the trade-offs between the benefits on one hand, and the risks, burden, and costs on the other.
For each recommendation the quality of evidence has been graded as:
A (high)
B (moderate)
C (low)
D (very low)
Grade A evidence means high quality evidence that comes from consistent results from well performed randomised controlled trials, or overwhelming evidence of another sort (such as well-executed observational studies with very strong effects).
Grade B evidence means moderate quality evidence from randomised trials that suffer from serious flaws in conduct, consistency, indirectness, imprecise estimates, reporting bias, or some combination of these limitations, or from other study designs with special strength.
Grade C evidence means low quality evidence from observational evidence, or from controlled trials with several very serious limitations.
Grade D evidence is based only on case studies or expert opinion.
A Level 1 recommendation is a strong recommendation to do (or not to do) something where the benefits clearly outweigh the risks (or vice versa) for most, if not all patients.
A Level 2 recommendation is a weaker recommendation, where the risks and benefits are more closely balanced or are more uncertain.
Abbreviations
CMV Cytomegalovirus
ISDR Immunosuppression Dose Reduction
RCT Randomised Controlled Trial
SOT Solid Organ Transplant
Definitions
CMV infection: evidence of the virus undergoing a complete replication cycle and producing new infectious virions. This may be further characterised as:
- Asymptomatic infection (no obvious signs of pathologic symptoms)
- Viral syndrome (fever, leucopenia, myalgia or arthralgia)
- CMV disease – (histopathological evidence of CMV, CMV retinitis diagnosed by an ophthalmologist, or CMV in the CSF indicative of CNS disease)
Latent CMV: A state of virus infection in which the full replication cycle of the virus is not occurring
Serostatus: The presence or absence of detectable antibodies against a specific antigen, as measured by a blood test (serology test). Donor: recipient CMV serostatus is characterised as:
- Both donor and recipient are IgG antibody seropositive:
- Donor (D) positive (+); Recipient (R) positive (+) (D+/R+)
- Both donor and recipient are IgG antibody seronegative:
- Donor (D) negative (-); Recipient (R) negative (-) (D-/R-)
- Donor is IgG antibody seropositive and recipient is IgG antibody seronegative:
- Donor (D) positive (+); Recipient (R) negative (-) (D+/R-)
- Donor is IgG antibody seronegative and recipient is IgG antibody seropositive:
- Donor (D) negative (-); Recipient (R) positive (+) (D-/R+)
Disclaimer
This document provides a guide to best practice, which inevitably evolves over time. All clinicians involved in these aspects of transplantation need to undertake clinical care on an individualised basis and keep up to date with changes in the practice of clinical medicine.
These guidelines represent the collective opinions of a number of experts in the field and do not have the force of law. They contain information/guidance for use by practitioners as a best practice tool. It follows that the guidelines should be interpreted in the spirit rather than the letter of their contents. The opinions presented are subject to change and should not be used in isolation to define the management for any individual patient.
The guidelines are not designed to be prescriptive, nor to define a standard of care. The British Transplantation Society cannot attest to the accuracy, completeness or currency of the opinions contained herein and do not accept responsibility or liability for any loss or damage caused to any practitioner or any third party as a result of any reliance being placed on the guidelines or as a result of any inaccurate or misleading opinion contained in the guidelines.
References
- Zuhair M, Smit S, Wallis G, Jabbar F, Smith C, B, Griffiths P Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Reviews in Medical Virology 2019; 29: 2034
- Wentworth BB, Alexander ER. Sero epidemiology of infections due to members of the herpes virus group. Am J Epidemiol 1971; 94: 496-507.
- Gane E, Saliba F, Valdecasas GJ, et al. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplant Study Group. Lancet 1997; 350: 1729-33. Erratum in: Lancet 1998; 351: 454.
- Patel R, Snydman DR, Rubin RH, et al. Cytomegalovirus prophylaxis in solid organ transplant recipients. Transplantation 1996; 61: 1279-89.
- Harvala H, Stewart C, Muller K, Burns S, Marson L, MacGilchrist A, Johannessen I. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol 2013;85:893-8
- Mendez-Eirin E, Paniagua-Martín MJ, Marzoa-Rivas R, Barge-Caballero E, Grille-Cancela Z, Cañizares A, NayaLeira C, Gargallo-Fernández P, Castro-Beiras A, CrespoLeiro M. Cumulative incidence of cytomegalovirus infection and disease after heart transplantation in the last decade: effect of preemptive therapy. Transplant Proc 2012;44:2660-2
- Hammond SP, Martin ST, Roberts K, Gabardi S, Fuhlbrigge AL, Camp PC, Goldberg HJ, Marty FM, Baden LR. Cytomegalovirus disease in lung transplantation: impact of recipient seropositivity and duration of antiviral prophylaxis. Transpl Infect Dis 2013;15:163-70.
- Eid AJ, Razonable RR. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs 2010;70:965-81. 11
- Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, Razonable RR. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Inf Dis 2008;46:840-6.
- Snydman, DR , Werner BG, Heinze-Lacey B, et al. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med 1987; 317: 1049-54.
- Rubin RH. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA 1989; 261: 3607-9
- Lowance D, Neumayer HH, Legendre CM, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N Engl J Med 1999; 340: 1462-70.
- Humar A, Gillingham KJ, Payne WD, et al. Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation 1999; 68: 1879-83.
- Evans PC, Soin A, Wreghitt TG, et al. An association between cytomegalovirus infection and chronic rejection after liver transplantation. Transplantation 2000; 69: 30-5.
- Johnson RJ, Clatworthy MR, Birch R, Hammad A, Bradley JA. CMV mismatch does not affect patient and graft survival in UK renal transplant recipients. Transplantation 2009; 88: 77-82.
- Snydman DR, Werner BG, Heinze-Lacey B, et al. Use of cytomegalovirus immuneglobulin to prevent cytomegalovirus disease in renal transplant recipients. N Engl J Med 1987; 317: 1049-54.
- Snydman DR, Werner BG, Dougherty NN, et al. Cytomegalovirus immune globulin prophylaxis in liver transplantation. Ann Intern Med 1993; 119: 984-91.
- Bonaros N, Mayer B, Schachner T, Laufer G, Kocher A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant 2008;22:89–97
- Vrtovec, B.; Thomas, C. D.; Radovancevic, R.; Frazier, O. H.; Radovancevic, B. Comparison of intravenous ganciclovir and cytomegalovirus hyperimmune globulin pre-emptive treatment in cytomegalovirus-positive heart transplant recipients. Journal of Heart & Lung Transplantation. 2004;23(4):461-5
- Crumpacker C, Marlowe S, Zhang JL, et al. Treatment of cytomegalovirus pneumonia. Rev Infectious Dis 1988; 10(S3): 538-46.
- Dunn DL, Mayoral JL, Gillingham KJ, et al. Treatment of invasive cytomegalovirus disease in solid organ transplant patients with ganciclovir. Transplantation 1991; 51: 98-106.
- Harbison MA, De Girolami PC, Jenkins RL, Hammer SM. Ganciclovir therapy of severe cytomegalovirus infections in solid-organ transplant recipients. Transplantation 1988; 46: 82-8.
- de Koning J, van Dorp WT, van Es LA van‘t Wout JW, van der Woude FJ. Ganciclovir effectively treats cytomegalovirus disease after solid-organ transplantation, even during rejection treatment. Nephrol Dial Transplant 1992; 7:350-6.
- George MJ, Snydman DR, Werner BG, et al. Use of ganciclovir plus cytomegalovirus immune globulin to treat CMV pneumonia in orthotopic liver transplant recipients. Transplant Proc 1993; 25: 22.
- Falgas ME, Snydman DR, Griffith J, et al. Clinical and epidemiological predictors of recurrent cytomegalovirus disease in orthotopic liver transplant recipients. Clin Infect Dis 1997; 25: 314-7.
- van den Berg AP, van Son WJ, Haagsma EB, et al. Prediction of recurrent cytomegalovirus disease after treatment with ganciclovir in solid-organ transplant recipients. Transplantation 1993; 55: 847-51.
- Gould FK, Freeman R, Taylor CE, et al. Prophylaxis and management of cytomegalovirus pneumonitis after lung transplantation: a review of experience in one center. J Heart Lung Transplant 1993; 12: 695-9.
- Kirklin JK, Naftel DC, Levine TB, et al. Cytomegalovirus after heart transplantation. Risk factors for infection and death: a multi-institutional study. J Heart Lung Transplant 1994; 13: 394-404.
- Manez R, Kusne G, Green M, et al. Incidence and risk factors associated with the development of cytomegalovirus disease after intestinal transplantation. Transplantation 1995; 59: 1010-4.
- Gardiner BJ, Chow JK, Price LL, Nierenberg NE, Kent DM, Snydman DR. Role of Secondary Prophylaxis With Valganciclovir in the Prevention of Recurrent Cytomegalovirus Disease in Solid Organ Transplant Recipients. Clin Infect Dis 2017; 65(12):2000-2007
- Nichols WG, Corey L, Gooley T, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood 2001;97: 867-74.
- Chou SW. Cytomegalovirus drug resistance and clinical implications. Transpl Infect Dis 2001: 3(S2): 20-4.
- Ronak G Gandhi. Evaluating the Safety of Maribavir for the Treatment of Cytomegalovirus. Therapeutics and Clinical Risk Management 2022:18 223–232
- NICE Technology Appraisal TA860: Maribavir for treating refractory or resistant cytomegalovirus infection after transplant. Published January 2023
- BTS Guideline Development Policy 2021. Accessed at https://bts.org.uk/wp-content/uploads/2021/05/BTS_Guideline_Development_Policy_2021.pdf
- https://casp-uk.net/casp-tools-checklists/
- Atkins D, Best D, Briss PA, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. Br Med J 2004; 328: 1490.
- Uhlig K, Macleod A, Craig J, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 70: 2058-65.
- Kidney Disease Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(S3): S1-157.
Rationale for Clinical Practice Recommendations for the Prevention and Management of CMV following solid organ transplantation
1 Preventing CMV Infection and Disease
Determining CMV status in donor and recipient
1.1 All organ donors and recipients should be screened for CMV (IgG antibody) serostatus prior to, or at the time of transplantation [1A]
1.2 If donor CMV serostatus is unknown and recipient is seronegative or unknown for CMV, this should be characterised as donor seropositive, recipient seronegative for CMV (D+/R-). [1D]
CMV prophylaxis
For adults, children and young people receiving a solid organ transplant:
1.3 Offer Valganciclovir prophylaxis to people receiving kidney and liver transplants for at least 3 months following transplantation if either:
- The recipient is seronegative for CMV and receives an allograft from a CMV seropositive donor (D+/R-) [1A] OR
- The recipient has received T-lymphocyte depletion therapy with Antithymocyte gobulin (ATG) or Alemtuzumab (Campath®), where donor: recipient serostatus is D+/R+ or D-/R+ [1A]
1.4 Consider Valganciclovir prophylaxis for people receiving heart, lung, intestinal or pancreas transplants for at least 3 months following transplantation if either:
- The recipient is seronegative for CMV and receives an allograft from a CMV seropositive donor (D+/R-) [1C] OR
- The recipient has received T-lymphocyte depletion therapy with Antithymocyte gobulin (ATG) or Alemtuzumab (Campath®), where donor: recipient serostatus is D+/R+ or D-/R+ [1C]
1.5 Do not routinely offer Valganciclovir prophylaxis if donor and recipient are seronegative for CMV (D-/R-) [1D]
1.5.1 Be aware that some recipients who are seronegative for CMV (D-/R-) may require Valganciclovir or Valaciclovir prophylaxis for prevention of other Herpes virus infections, e.g; recipients who are seronegative for Herpes Simplex Virus (HSV) receiving an organ from HSV seropositive donors [2D].
1.6 Consider Valganciclovir prophylaxis for at least 3 months after starting treatment for acute allograft rejection if either donor or recipient are CMV positive (D+/R-, D+/R+ or D-/R+) [2C].
1.7 Adjust the dose of Valganciclovir if creatinine clearance is less than 60mL/minute [1D]
1.8 For children and young people under 18 years, adjust the dose of Valganciclovir according to body surface area, up to a maximum dose of 900mg once daily [1D]
1.9 Monitor Full Blood Count at least every 2 weeks whilst on Valganciclovir [1A]
1.10 Consider switching to CMV monitoring and pre-emptive therapy if people develop side effects e.g.; neutropenia, on Valganciclovir [1C]
Research recommendations:
In adults, children and young people receiving solid organ transplants:
- What is the clinical and cost-effectiveness of anti-CMV prophylaxis vs active surveillance (monitoring followed by pre-emptive therapy), for people receiving heart, lung, liver, intestinal or pancreas transplants for at least 3 months following transplantation?
- What is the clinical and cost-effectiveness of anti-CMV prophylaxis vs active surveillance (monitoring followed by pre-emptive therapy), following treatment of CMV infection or disease?
In children and young people receiving solid organ transplants:
- What is the clinical and cost-effective calculation for dosing Valganciclovir according to body surface area?
Audit measures:
- Proportion of adults, children and young people receiving solid organ transplants who are offered Valganciclovir prophylaxis according to donor-recipient CMV profiles and use of T-cell depletion therapy as set out in recommendation 1.3
Rationale
The risk of developing CMV infection or disease following solid organ transplantation is defined on the basis of donor and recipient CMV status prior to transplantation, as expressed in this and other published guidelines [1-3]. Pretransplant screening for CMV is therefore widely agreed to be a prerequisite for transplantation and evidence suggests that specific CMV IgG serology status should be ascertained in donor and recipient [4].
The committee agreed that, where donor CMV status is unknown or indeterminate, the donor should be assumed to be seropositive so that the highest possible risk profile is assumed based on the results available [1, 5]. If the recipient status is also unknown or indeterminate, the donor / recipient profile should be ascribed a CMV status of high risk (D+/R-). The committee acknowledged the low-quality evidence, but felt, on balance that this approach will reduce the risk of primary CMV infection or reactivation in people at high or intermediate risk of harm from CMV disease [6-10].The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO) recommends that donor CMV serostatus should be established within 30 days of organ donation [11]. The committee agreed that unknown CMV donor status should be clarified as soon as possible, to minimise unnecessary pharmaco-prophylaxis. The committee also noted that prospective SOT donors and recipients who are CMV seronegative may become positive with time and consideration should be given to repeating CMV serology at the time of transplant for seronegative recipients and donors.
The committee was satisfied that there is sufficient high-quality evidence to offer CMV prophylaxis with Valganciclovir for 3 months to people in D+/R- donor-recipient category or following T-cell depletion therapy in all categories other than D-/R- following kidney transplantation [9, 10, 12that health professionals consider CMV prophylaxis with Valganciclovir for 3 months following transplantation in these other SOT recipients. In arriving at this decision, members acknowledged that some centres have excellent results from monitoring and pre-emptive therapy, but noted that the ability to undertake the monitoring required is likely to vary between centres.
The committee acknowledged the equipoise between CMV prophylaxis and monitoring with pre-emptive treatment for D+/R+ and D-/R+ donor-recipient categories, with moderate quality evidence for both approaches, and agreed that either could be recommended [13-15]. The committee acknowledged variation in practice between some units with regard to offering Valganciclovir in D-/R+ donor-recipient categories, however, viraemia has been noted in up to 40% of SOT transplants in this category, many of whom have required treatment [13].
In making these decisions, health professionals should take into account patient preference, tolerance of medications, and adherence with the selected strategy, and logistics associated with surveillance and pre-emptive strategy.
Valganciclovir dosing should follow recommended licensed dosing from the drug company. For children and young people (CYP) under the age of 18, Valganciclovir dosing is adjusted according to body surface area (BSA) and is used ‘off-label’. The committee noted that there is no evidence to support a specific algorithm for calculating dose adjustments in CYP and some variation in practice exists as a result of the availability of different dose-adjustment calculations. The committee agreed that Valganciclovir dosing in CYP should be limited to a maximum dose of 900mg once and that a research recommendation should be made to ascertain the most clinically and cost-effective dosing strategy for Valganciclovir in CYP.
There is emerging evidence that low dose (450mg) Valganciclovir prophylaxis is as effective as standard dose (900mg) in kidney transplant recipients [17, 18], however, the development of Ganciclovir resistance is not well described in these studies and low dose Valganciclovir prophylaxis is not currently licensed in the UK.
Strong associations between acute allograft rejection and late onset CMV disease were reported in early studies [19-21] and the committee agreed to make a recommendation to consider Valganciclovir prophylaxis for 3 months to people at risk of CMV disease following acute allograft rejection and based on serostatus (D+/R-, D+/R+ or D-/R+). The committee noted existing guidance recommending antimicrobial prophylaxis for opportunistic infection when treating rejection with high dose steroids or cytolytic therapy in recipients receiving heart transplants [22] and further acknowledged variation in practice across different transplant centres.
The committee did not make any recommendations on secondary prophylaxis following treatment of CMV infection or disease in the absence of evidence and lack of consensus within the group to support this; a research recommendation was agreed.
References
- Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102(6):900-31.
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13512.
- Girmenia C, Lazzarotto T, Bonifazi F, Patriarca F, Irrera G, Ciceri F, et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: A multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin Transplant. 2019;33(10):e13666.
- Delforge ML, Desomberg L, Montesinos I. Evaluation of the new LIAISON(®) CMV IgG, IgM and IgG Avidity II assays. J Clin Virol. 2015;72:42-5.
- Fishman JA, Emery V, Freeman R, Pascual M, Rostaing L, Schlitt HJ, et al. Cytomegalovirus in transplantation – challenging the status quo. Clin Transplant. 2007;21(2):149-58.
- Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006;6(9):2134-43.
- Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338(24):1741-51.
- Kotton CN. CMV: Prevention, Diagnosis and Therapy. Am J Transplant. 2013;13 Suppl 3:24-40; quiz
- Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4(4):611-20.
- Varela-Fascinetto G, Benchimol C, Reyes-Acevedo R, Genevray M, Bradley D, Ives J, et al. Tolerability of up to 200 days of prophylaxis with valganciclovir oral solution and/or film-coated tablets in pediatric kidney transplant recipients at risk of cytomegalovirus disease. Pediatr Transplant. 2017;21(1).
- https://www.gov.uk/government/publications/sabto-report-of-the-cytomegalovirus-steering-group
- Raval AD, Kistler K, Tang Y, Murata Y, Snydman DR. Antiviral treatment approaches for cytomegalovirus prevention in kidney transplant recipients: A systematic review of randomized controlled trials. Transplant Rev (Orlando). 2021;35(1):100587
- S F Atabani, C Smith, C Atkinson, R W Aldridge, M Rodriguez-Perálvarez, N Rolando, M Harber, G Jones, A O’Riordan, A K Burroughs, D Thorburn, J O’Beirne, R S B Milne, V C Emery, P D Griffiths. Kinetics in solid organ transplant recipients managed by preemptive therapy Am J Transplant 2012 Sep;12(9):2457-64.
- Florescu DF, Qiu F, Schmidt CM, Kalil AC. A direct and indirect comparison meta-analysis on the efficacy of cytomegalovirus preventive strategies in solid organ transplant. Clin Infect Dis. 2014;58(6):785-803.
- Singh N, Winston DJ, Razonable RR, Lyon GM, Silveira FP, Wagener MM, Limaye AP. Cost effectiveness of preemptive therapy versus prophylaxis in a randomized clinical trial for the prevention of CMV disease in seronegative liver transplant recipients with seropositive donors. Clin Infect Dis. 2020:ciaa1051. doi: 10.1093/cid/ciaa1051
- Manuel O, Kralidis G, Mueller NJ, Hirsch HH, Garzoni C, van Delden C, et al. Impact of antiviral preventive strategies on the incidence and outcomes of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2013;13(9):2402-10.
- Hwang SD, Lee JH, Lee SW, Kim JK, Kim MJ, Song JH. Effect of Low-Dose Vs Standard-Dose Valganciclovir in the Prevention of Cytomegalovirus Disease in Kidney Transplantation Recipients: A Systemic Review and Meta-Analysis. Transplant Proc. 2018;50(8):2473-2478
- Xin W, Hui Y, Xiaodong Z, Xiangli C, Shihui W, Lihong L. Effectiveness of Valganciclovir 900mg Versus 450mg for Cytomegalovirus Prophylaxis in Renal Transplantation: A Systematic Review and Meta-Analysis. J Pharm Pharm Sci. 2017;20(0):168-183
- RennieTJ, Geddes CG, McIntyre-McClure R, Chua BE, Metcalfe W, Johannessen I, Phelan On behalf of the SCOT-network C. M. V. prophylaxis working group. Efficacy and side effect profile of two CMV prophylaxis strategies in high and intermediate risk kidney transplant recipients – a multicentre national study. J Nephrol 2021 Dec;34(6):2173-2175.
- Razonable RR, Rivero A, Rodriguez A, et al. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxiswith oral ganciclovir. J Infect Dis. 2001;184:1461–1464.
- Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10:1228–1237
- The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients: Practice Guideline. J Heart Lung Transplant. 2010 Aug;29(8):914-56.
2 Laboratory Testing for CMV
2.1 Clinical Laboratories should use Quantitative Nucleic Acid Testing (QNAT) of whole blood or plasma to quantify CMV viral load [1A]
- Transplant centres should adopt a consistent approach to use either whole blood or plasma for quantification of CMV viral load [1C]
- CMV QNAT assays should be calibrated using the WHO International Reference Standard [1C]
- CMV viral load should be reported as IU/mL, where possible, or, if reported as copies / mL, a conversion factor should be used [1C]
2.2 Transplant centres should determine local thresholds of viral load that portend CMV infection or disease requiring treatment, depending on the CMV QNAT assay they use and the population at risk [1C]
2.3 QNAT thresholds should not be used in isolation to determine commencement or cessation of treatment for individuals with CMV disease, but should be considered in the context of clinical, laboratory or histological evidence of CMV disease [1C]
Audit measures:
- Proportion of Clinical Laboratories using Quantitative Nucleic Acid Testing (QNAT) of whole blood or plasma to quantify CMV viral load
- Proportion of CMV QNAT assays calibrated using the WHO International Reference Standard.
Rationale
There is high quality evidence to support the use of quantitative nucleic acid testing methods (QNAT) for surveillance or diagnostic CMV testing of recipients in the post-transplant phase. The QNAT method quantitates the amount of viral nucleic acid (DNAaemia, or viral load) present within a clinical specimen, utilising polymerase chain reaction (PCR) methods [1, 2]. QNAT can guide the initiation of therapy and allow assessment of the response to treatment [3]. In a randomised trial that compared QNAT with pp65 antigenaemia CMV surveillance in solid organ transplant recipients, QNAT resulted in fewer patients initiating treatment, but the incidence of CMV disease was the same in both groups [4]. A drawback of the QNAT method is that inter-assay variability exists, which limits the ability to compare results between laboratories [1]. Laboratories may either perform QNAT on whole blood or plasma, however, the committee noted the importance of being consistent with the approach selected, in order to allow accurate monitoring with serial testing. Higher levels of CMV viral load have been reported when whole blood rather than plasma is used for QNAT testing [5]. The likely reason for this is that on analysis of the plasma, only extracellular viral material is detected, whereas intracellular material is additionally detected in whole blood. The committee noted variation between centres in reporting results and made a recommendation to provide a conversion factor when reporting as copies / mL to facilitate greater consistency of results. Given the high inter-assay and inter laboratory variability of results provided by QNAT [6], individual transplant centres must select the threshold for initiation and cessation of anti-CMV treatment. This should be based on knowledge of the assay being carried out in the laboratory and expert opinion. Even within a single laboratory, utilising the same assay, variability in quantitative CMV viral load is to be expected. The extent of variability differs depending on the level of viral load, with greater variability at lower levels (<1000 IU/mL) [7]. The committee was not able to make any recommendation on what change in viral load is considered clinically significant. International guidelines recommend that a change of 0.5log (approximately 3-fold) be considered as a clinically significant, as this has been reported to be the upper limit of assay variability [7, 8].
In asymptomatic solid organ transplant recipients undergoing CMV surveillance, the initiation and cessation of anti-CMV treatment is primarily influenced by the viral load detected through QNAT of blood or plasma. The committee agreed that, in individuals with clinical manifestations suggestive of CMV disease, the CMV QNAT result should not be used in isolation to guide anti-CMV therapy. This is because the level of viral load in the blood compartment may not correlate with the severity of tissue invasive disease and QNAT of plasma or blood may not be the best indicator of disease resolution [9]. Further diagnostic testing of solid organ transplant recipients suspected of having CMV disease needs to be individualised and should be guided by clinicians with expertise in this area.
References
- Hirsch HH, Lautenschlager I, Pinsky BA, Cardeñoso L, Aslam S, Cobb B, et al. An international multicenter performance analysis of cytomegalovirus load tests. Clin Infect Dis. 2013;56(3):367-73.
- Fryer JF HA AR, Minor PD, and the collaborative study group. Collaborative study to evaluate the proposed 1st WHO International Standard for human cytomegalovirus (HCMV) for nucleic acid amplification (NAT)-based assays. . WHO; 2010.
- Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev. 2013;26(4):703-27.
- Gerna G, Baldanti F, Torsellini M, Minoli L, Viganò M, Oggionnis T, et al. Evaluation of cytomegalovirus DNAaemia versus pp65-antigenaemia cutoff for guiding preemptive therapy in transplant recipients: a randomized study. Antivir Ther. 2007;12(1):63-72.
- Lisboa LF, Asberg A, Kumar D, Pang X, Hartmann A, Preiksaitis JK, et al. The clinical utility of whole blood versus plasma cytomegalovirus viral load assays for monitoring therapeutic response. Transplantation. 2011;91(2):231-6.
- Preiksaitis JK, Hayden RT, Tong Y, Pang XL, Fryer JF, Heath AB, et al. Are We There Yet? Impact of the First International Standard for Cytomegalovirus DNA on the Harmonization of Results Reported on Plasma Samples. Clin Infect Dis. 2016;63(5):583-9.
- Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102(6):900-31.
- Abbate I, Piralla A, Calvario A, Callegaro A, Giraldi C, Lunghi G, et al. Nation-wide measure of variability in HCMV, EBV and BKV DNA quantification among centers involved in monitoring transplanted patients. J Clin Virol. 2016;82:76-83.
- Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2010;10(1):157-61.
3 Monitoring CMV Infection and Disease
3.1 Offer Monitoring of CMV viral load to guide pre-emptive therapy to adults, children and young people receiving a solid organ transplant if:
- They are CMV seropositive (D+/R+, D-/R+) [1B] AND
- They have not received T-lymphocyte depletion therapy [1B] AND
- They are not receiving valganciclovir prophylaxis [1B] (see section 1)
3.2 Where CMV viral load monitoring is offered:
- Consider testing with a frequency of at least monthly [1D]
- Consider testing for at least 3 months following transplantation [1D]
Research recommendations
In adults, children and young people receiving solid organ transplants:
- What is the most clinical and cost-effective duration and frequency of CMV viral load monitoring?
- What is the clinical and cost-effectiveness of CMV viral load monitoring following cessation of anti CMV prophylaxis?
- What is the clinical and cost-effectiveness of CMV viral load monitoring following completion of treatment of CMV infection or disease?
- What is the clinical and cost-effectiveness of CMV immune monitoring?
Audit Measures
- Proportion of adults, children and young people characterised in recommendation 3.1 who are offered monthly CMV viral load monitoring for 3 months following solid organ transplantation
Rationale
The decision to monitor for CMV viral load following solid organ transplantation should take into account risk factors for CMV disease, including donor-recipient CMV serostatus, treatment with T-lymphocyte depletion therapy and the use of Valganciclovir prophylaxis. Clinicians should also take note of patient preference and logistical issues such as frequency of post-transplant clinic follow up and turnaround time for CMV test results.
Members of the guideline committee agreed that recipients of solid organ transplants at highest risk of developing CMV disease should receive antiviral prophylaxis as set out in section 1.3 and that monitoring during this period of prophylaxis is not warranted since this is neither supported by evidence nor by group consensus.
The committee considered that CMV viral load monitoring in the early post-transplant period for people with D+/R+ and D-/R+ serostatus who are not receiving antiviral prophylaxis, is appropriate, despite limited quality and quantity of evidence. One RCT subjected 455 renal transplant recipients to weekly CMV pp65 antigenaemia testing. Those with a positive test were randomised to pre-emptive ganciclovir or deferred treatment. Significantly more patients in the control group developed CMV disease, suggesting that monitoring for pre-emptive treatment demonstrates benefit in this population, however, only 80 patients completed the study [1]. No randomised or comparative studies duration or frequency of monitoring in different patient groups were identified. Members agreed that CMV viral load monitoring at monthly intervals for the first 3 months following transplantation would be a pragmatic approach, but made a research recommendation to assess the clinical and cost-effectiveness of this strategy. Members also considered whether this recommendation should be restricted to D+/R+ recipients, but noted that CMV viremia has been noted in up to 40% of D-/R+ recipients with a third of these requiring treatment for CMV (T Haque, personal communication).
No evidence was identified to support CMV viral load monitoring in D-/ R- recipients during the initial post-transplant period, hence the committee did not recommend routine monitoring for this group.
The committee could not make a clear recommendation to support CMV viral load monitoring following cessation of prophylaxis, including after treatment for acute allograft rejection or following treatment of CMV disease. The committee acknowledged the risk of late onset CMV disease, reported in up to 30% of people receiving SOT [2-6]. The committee noted that CMV monitoring following cessation of prophylaxis is recommended in other national clinical guidance [7-10]. However, there is no robust evidence that this improves outcomes or reduces the risk of CMV disease [11-13]. The committee agreed to make a research recommendation.
The committee reviewed a number of case series investigating the role of immune monitoring (i.e.; CMV-specific T-cell immunity) to guide prophylaxis and/or initiation of pre-emptive therapy [14-18]. Members agreed that, whileimmune monitoring may in future be a useful adjunct to guide initiation and duration of prophylaxis and frequency of viral load monitoring, there was neither sufficient evidence nor clinical consensus to support a clear recommendation at this stage. The committee agreed that this issue warrants further research.
Figure 1 summarises the pathway for CMV monitoring and prophylaxis based on the donor and recipient serostatus.
References
- Sagedal S, Nordal KP, Hartmann A, Midtvedt K, Foss A, Asberg A, et al. Pre-emptive therapy of CMVpp65 antigen positive renal transplant recipients with oral ganciclovir: a randomized, comparative study. Nephrology Dialysis Transplantation. 2003;18(9): 1899-908.
- Meesing A, Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs. 2018;78(11):1085‐
- Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4(4):611‐
- Razonable RR, Blumberg EA. It’s not too late: a proposal to standardize the terminology of “late‐onset” cytomegalovirus infectionand disease in solid organ transplant recipients. Transpl Infect Dis. 2015;17(6):779‐
- Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxisin high‐risk kidney transplant recipients. Am J Transplant 2010;10(5):1228‐
- Humar A, Limaye AP, Blumberg EA, et al. Extended valganciclovir prophylaxis in D+/R‐ kidney transplant recipients is associated with long‐term reduction in cytomegalovirus disease: two‐year results of the IMPACT study. Transplantation. 2010;90(12):1427‐
- Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A. The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018 Jun;102(6):900-931. https://pubmed.ncbi.nlm.nih.gov/29596116/
- Girmenia C, Lazzarotto T, Bonifazi F, Patriarca F, Irrera G, Ciceri F, et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: a multidisciplinary consensus conference by the Italian GITMO, SITO and AMCLI societies. Clinical Transplantation. 2019;33(10):e13666. https://dx.doi.org/10.1111/ctr.13666
- Razonable RR, Humar A. Cytomegalovirus in Solid Organ Transplant Recipients – Guidelines of the American Society of Transplantation Infectious Disease Community of Practice. Clinical Transplantation. 2019;33(9):e13512.
- Schaenman J, Phonphok K, Spanuchart I, Duong T, Sievers TM, Lum E, et al. Early cytomegalovirus DNAemia and antiviral dose adjustment in high vs intermediate risk kidney transplant recipients. Transpl Infect Dis. 2021;23(1):e13457.
- Boillat Blanco N, Pascual M, Venetz JP, Nseir G, Meylan PR, Manuel O. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation. 2011;91(2):251-5.
- Chaiyapak T, Borges K, Williams A, Banh T, Vasilevska-Ristovska J, Allen U, et al. Incidence of Cytomegalovirus DNAemia in Pediatric Kidney Transplant Recipients After Cessation of Antiviral Prophylaxis. Transplantation. 2018;102(8):1391-6.
- Cunha L, Laranjinha I, Birne R, Jorge C, Carvalho TJ, Lanca A, et al. Late Cytomegalovirus Infection in Kidney Transplant Recipients after a Six-Month Prevention Protocol. Int. 2019;10(1):1-12. https://pubmed.ncbi.nlm.nih.gov/30891165/
- Thompson G, Boan P, Baumwol J, Chakera A, MacQuillan G, Swaminathan S, et al. Analysis of the QuantiFERON-CMV assay, CMV viraemia and antiviral treatment following solid organ transplantation in Western Australia. Pathology. 2018;50(5):554-61.
- Schachtner T, Stein M, Reinke P. CMV-Specific T Cell Monitoring Offers Superior Risk Stratification of CMV-Seronegative Kidney Transplant Recipients of a CMV-Seropositive Donor. Transplantation. 2017;101(10):e315-e25.
- Rogers R, Saharia K, Chandorkar A, Weiss ZF, Vieira K, Koo S, et al. Clinical experience with a novel assay measuring cytomegalovirus (CMV)-specific CD4+ and CD8+ T-cell immunity by flow cytometry and intracellular cytokine staining to predict clinically significant CMV events. BMC Infect Dis. 2020;20(1):58.
- Paez-Vega A, Gutierrez-Gutierrez B, Aguera ML, Facundo C, Redondo-Pachon D, Suner M, et al. Immunoguided Discontinuation of Prophylaxis for Cytomegalovirus Disease in Kidney Transplant Recipients Treated with Antithymocyte Globulin: A Randomized Clinical Trial. Clin Infect Dis. 2021;22:22.
- Meesing A, Abraham RS, Razonable RR. Clinical Correlation of Cytomegalovirus Infection With CMV-specific CD8+ T-cell Immune Competence Score and Lymphocyte Subsets in Solid Organ Transplant Recipients. Transplantation. 2019;103(4):832-8.
4 Treating CMV Infection and Disease
For adults, children and young people who develop CMV infection or disease following solid organ transplantation:
4.1 Offer treatment with oral Valganciclovir for a duration of at least 2 weeks [1A].
4.2 Adjust the dose of Valganciclovir according to licensed dosing recommendations if creatinine clearance is less than 60mL/minute [1D]
4.3 Be aware of the potential development of ganciclovir resistance (see section 5 for management)
4.4 Assess CMV viral load after 2 weeks of treatment and repeat at a minimum interval of 7 days [1D] AND
- Consider stopping treatment for CMV disease after resolution of symptoms AND two consecutive, CMV viral load tests that confirm that CMV is not detected (below the local laboratory threshold for detection) [2D].
Research recommendations
In adults, children and young people receiving solid organ transplants:
- What is the clinical and cost-effectiveness of using CMV viral load (QNAT) testing to guide cessation of treatment of CMV infection and disease?
- What is the clinical and cost-effectiveness of CMV specific immunoglobulins and CMV specific cytotoxic T cells in the treatment of severe, resistant or refractory CMV disease?
Audit Measures
- Proportion of adults, children and young people who are offered Valganciclovir to treat CMV infection and disease following solid organ transplantation
Rationale
The committee was satisfied that there is high quality evidence that oral valganciclovir is as effective as intravenous ganciclovir in treating CMV infection and disease following SOT. [1-4]. Most commonly valganciclovir 900mg twice daily has been used as treatment dose, adjusted for level of kidney function. Valganciclovir has added advantages of not requiring hospitalisation or intravenous cannulation [1]. However, the committee agreed that intravenous Ganciclovir would be the preferred treatment if concern exists regarding enteric absorption and the subsequent oral bioavailability of medications. The committee also agreed that, in very sick people requiring hospitalisation and other intravenous therapies e.g.; intravenous (i.v.) fluids and antibiotics, i.v ganciclovir should be considered due to a likelihood of impaired absorption. Based on knowledge of the viral kinetics with anti-CMV treatment, members agreed to recommend treatment for at least 14 days duration as this has been shown to be associated with a viraemia reduction of approximately 1.0 log10 (90%) [1, 2]. However, most clinical trials have had a longer duration of treatment and the VICTOR trial reported a 60% chance of ongoing viraemia at day 14 of treatment [1]. Therefore, the committee agreed to make a recommendation to repeat CMV viral load (QNAT) after 14 days of treatment and then at subsequent intervals of no shorter than 7 days, with consideration of cessation of anti-CMV treatment following two consecutive negative QNAT tests and clinical resolution of CMV disease. The committee acknowledged that, in a small number of cases, there may be ongoing, low level viral replication and clinicians should make a pragmatic decision with patients and / or carers about the time to stop treatment. Committee members also agreed that concerns of worsening CMV disease, would require earlier assessment of viral load and noted that indications for stopping treatment would be based on individual clinical risk assessments. The committee acknowledged that evidence supporting this recommendation is limited and agreed to make a research recommendation.
The dose of both Valganciclovir and Ganciclovir needs to be adjusted according to renal function, as these drugs are primarily cleared by the kidneys and accumulation may potentiate toxicity [5]. We recommend using licensed dosing for treatment and prophylaxis from the company with adjustments for renal function made according to the Renal Drug Handbook, using the Cockroft-Gault formula to calculate creatinine clearance. The Renal Drug Handbook also includes information on higher treatment doses, however, the committee acknowledged that this would only be considered by clinicians with the necessary experience and expertise.
The committee identified a single small RCT that evaluated pre-emptive treatment with CMV Immunoglobulin (CMV Ig) in 37 recipients of heart transplants, that reported similar efficacy of CMV Ig and intravenous ganciclovir in preventing CMV disease [6]. The committee was not persuaded that this was sufficient to make a recommendation on treatment with CMV Ig. No evidence was identified to support a recommendation on the use of adoptive transfer of cytotoxic T Lymphocytes (CTL) for pre-emptive treatment of CMV. The committee agreed to make research recommendation for both CMVIg and CTL.
References
- Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7(9):2106-13.
- Mattes FM, Hainsworth EG, Hassan-Walker AF, Burroughs AK, Sweny P, Griffiths PD, et al. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191(1):89-92.
- Singh N, Wannstedt C, Keyes L, Mayher D, Tickerhoof L, Akoad M, et al. Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts. Liver Transpl. 2008;14(2):240-4.
- Kalpoe JS, Schippers EF, Eling Y, Sijpkens YW, de Fijter JW, Kroes AC. Similar reduction of cytomegalovirus DNA load by oral valganciclovir and intravenous ganciclovir on pre-emptive therapy after renal and renal-pancreas transplantation. Antivir Ther. 2005;10(1):119-23.
- Perrottet N, Decosterd LA, Meylan P, Pascual M, Biollaz J, Buclin T. Valganciclovir in adult solid organ transplant recipients: pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements. Clin Pharmacokinet. 2009;48(6):399-418.
- Vrtovec, B.; Thomas, C. D.; Radovancevic, R.; Frazier, O. H.; Radovancevic, B. Comparison of intravenous ganciclovir and cytomegalovirus hyperimmune globulin pre-emptive treatment in cytomegalovirus-positive heart transplant recipients. Journal of Heart & Lung Transplantation. 2004;23(4):461-5
5 Ganciclovir resistance
Audit measures:
- Proportion of adults, children and young people with Ganciclovir resistance tested for UL97 and UL54 mutations.
Rationale
CMV disease that is refractory to treatment with Ganciclovir or Valganciclovir is termed Ganciclovir resistance and is reported to occur in up to 3% of SOT recipients [1, 2]. Ganciclovir resistance may be suspected in difficult to control CMV infection, as evidenced by a persistent or increasing viral load or symptomatic disease after a normally effective dosage and duration (e.g.; 2-4 weeks) of Ganciclovir or Valganciclovir [3]. Although evidence is limited, prolonged antiviral courses, sub-therapeutic antiviral dosing, intensive immunosuppression, and T-cell depletion treatment are associated with an increased risk for developing Ganciclovir resistance, particularly in a solid organ transplant recipient receiving an organ from a CMV seropositive donor [2, 4-6].
Ganciclovir resistance may be secondary to a viral genetic alteration that decreases susceptibility to one or more antiviral drugs (e.g.; UL97 and UL54,). CMV UL97 encodes for a viral kinase that catalyses the initial mono‐phosphorylation and activation of ganciclovir. In people receiving treatment with Valganciclovir (or Ganciclovir), UL97 kinase gene mutations are reported to appear first in around 90% of cases [5,7], with UL54 DNA polymerase gene mutations usually evolving later, conferring increased Ganciclovir resistance and likely cross-resistance to Cidofovir and Foscarnet [8]. In a post-hoc analysis of the VICTOR study (comparing Valganciclovir and intravenous Ganciclovir for the treatment of proven CMV disease), the presence of these resistance mutations significantly increased the risk of eradication failure at both day 21 (odds ratio [OR], 11.04; P = 0.02) and day 49 (6.82; P = .001). Significantly longer half-lives were seen in UL97 mutants that conferred ganciclovir resistance than wild-type strains, polymorphic variants and strains with mutations of unknown significance (P = .045) [8, 9].
Genotypic assays can, relatively rapidly, detect gene mutations that are associated with both high and low-grade resistance to ganciclovir, as well as other mutations that confer various degrees of resistance to Ganciclovir, Foscarnet and Cidofovir. The committee agreed that resistance testing should be performed by automated sequencing methods using genotypic assays to identify specific resistance mutations in the UL97 and UL54 genes, since these are the two most widely described causes of Ganciclovir resistance.
Newer agents Letermovir and Maribavir may be associated with less drug resistance than Ganciclovir. The use of Maribavir for treating refractory or resistant CMV infection following solid organ transplantation has been assessed in phase 3 studies and reported to be superior to Ganciclovir, Cidofovir and Foscarnet [10, 11]. Maribavir has Food and Drugs Administration (FDA) and MHRA [12] approval and is now recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of CMV infection and / or disease that are refractory (with or without resistance) to one or more prior therapies, including valganciclovir, ganciclovir, cidofovir and foscarnet in adults who have undergone a solid organ transplant [13]. Treatment duration may need to be individualised based on clinical characteristics. For children and young people, treatment with Maribavir would be outside of its marketing authorisation. The committee had agreed to review the outcome of this Technology Appraisal Guidance once published in January 2023 and therefore updated the guideline in May 2023 to include a recommendation on Maribavir for suspected or confirmed CMV resistance, as set out in the recommendations. Committee members noted the importance of reporting and suspected adverse reactions via the national reporting system (www.mhra.gov.uk/yellowcard).
The committee also agreed that consideration could be given to testing for Letermovir and Maribavir- resistant mutations [14,15], but again noted the importance of seeking specialist virology advice
References
- Myhre HA, Haug Dorenberg D, Kristiansen KI, et al. Incidence and outcomes of ganciclovir‐resistant cytomegalovirus infections in 1244 kidney transplant recipients. Transplantation. 2011;92(2):217‐
- Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23(4):689‐
- Chemaly RF, Chou S, Einsele H, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2018.
- Young Pg, Rubin J, Angarone M, et al. Ganciclovir‐resistant cytomegalovirus infection in solid organ transplant recipients: a single‐center retrospective cohort study. Transpl Infect Dis. 2016;18(3):390‐
- Fisher CE, Knudsen JL, Lease ED, et al. Risk factors and outcomes of ganciclovir resistant cytomegalovirus infection in solid organ transplant recipients. Clin Infect Dis. 2017;65:57–63.
- Hakki M, Chou S. The biology of cytomegalovirus drug resistance. Curr Opinion Infect Dis. 2011;24(6):605‐
- Lurain NS, Bhorade SM, Pursell KJ, et al. Analysis and characterization of antiviral drug-resistant cytomegalovirus isolates from solid organ transplant recipients. J Infect Dis. 2002;186(6):760–768.
- Asberg A, Jardine AG, Bignamini AA, et al. Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients. Am J Transplant. 2010;10:1881–1888
- Asberg A, Humar A, Rollag H, et al. Lessons learned from a randomized study of oral valganciclovir versus parenteral ganciclovir treatment of cytomegalovirus disease in solid organ transplant recipients: the VICTOR trial. Clin Infect Dis. 2016;62:1154–1160.
- Ronak G Gandhi. Evaluating the Safety of Maribavir for the Treatment of Cytomegalovirus. Therapeutics and Clinical Risk Management 2022:18 223–232
- Avery, RK, Alain S, Alexander BD. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial. Clin Infect Dis 2022 Sep 10;75(4):690-701
- EMC product characteristic: Livtenicity https://www.medicines.org.uk/emc/product/14375/smpc#gref
- NICE Technology Appraisal TA860: Maribavir for treating refractory or resistant cytomegalovirus infection after transplant. Published January 2023
- Chou S, Satterwhite LE, Ercolani RJ. New locus of drug resistance in the human cytomegalovirus UL56 gene revealed by in vitro exposure to letermovir and ganciclovir. Antimicrob Agents Chemother. 2018;62(9):pii: e00922‐
- Razonable RR. Drug‐resistant cytomegalovirus: clinical implications of specific mutations. Curr Opin Organ Transplant. 2018;23(4):388
6 Immunosuppression dose reduction
For adults, children and young people who develop CMV infection or disease (with or without leukopenia) following solid organ transplantation:
6.1 Consider a dose reduction of either calcineurin inhibitor or mycophenolate mofetil / azathioprine [1C]
- Consider preferentially reducing or stopping Mycophenolate Mofetil (MMF) or azathioprine if there is evidence of leukopenia [2C]
6.2 Discuss with patients, and, where appropriate, parents or carers, the risk of acute rejection with immunosuppression dose reduction [1D]
6.3 Review the dosing of immunosuppression following resolution of CMV infection or disease [1D]
Research recommendations:
- In adults, children and young people who develop CMV infection or disease (with or without leukopenia) following solid organ transplantation, is reduction of Calcineurin Inhibitors (CNIs) or proliferation inhibitors associated with better outcomes?
Audit measures:
- Proportion of adults, children and young people who develop CMV infection or disease (with or without leukopenia) following solid organ transplantation, and who reduce or stop Calcineurin Inhibitors (CNIs) and / or proliferation inhibitors
Rationale
T-cell mediated immunity, including virus specific cytotoxic lymphocytes is the body’s main defence to CMV reactivation and disease [1]. Immunosuppression required to prevent and treat allograft rejection, limits the body’s response to controlling CMV. There is low quality evidence that reduction in immunosuppression drug dosing is associated with improved outcomes for solid organ transplant recipients with CMV disease [2, 3]. However, CMV infection itself and a reduction in immune suppression may increase the risk of graft rejection. Reflecting this difficult balance, there is wide variation in current practice. Members of the committee agreed to highlight the importance of discussing the possibility of allograft rejection following reduction in immune suppression with people who develop CMV disease following SOT.
Committee members were not able to make a recommendation on reducing immune suppression during, or immediately following an episode of acute rejection and agreed upon the need to undertake individual risk assessments for the tapering of immune suppression. There was also insufficient evidence to recommend maintaining reduced doses of immune suppression following resolution of CMV infection or disease; members agreed that this should be individualised.
The committee agreed that a reduction in the dose of either Calcineurin Inhibitors (CNIs) or proliferation inhibitors (PIs) is appropriate for CMV infection or disease, but, in the presence of leukopenia a reduction or cessation of PI may be preferred. Noting the clinical equipoise, the committee agreed to make a research recommendation to further assess this.
There is low quality evidence that conversion to an mTOR-inhibitor based regimen may provide some protection against recurrent CMV [4-7]. However, the committee agreed that the quality of the evidence is insufficient to recommend conversion to an mTOR-inhibitor during or following CMV infection or disease.
References
- The immune response to human CMV. Corinna La Rosa and Don J Diamond* Future Virol. 2012 Mar 1; 7(3): 279–293.doi: 10.2217/fvl.12.8
- Anglicheau D, Lautrette A, Scieux C, Thervet E, et al. Lowering immunosuppression is safe and effective to treat altered pattern of CMV infection in renal transplant recipients on valaciclovir prophylaxis. Transplant Proc. 2002;34(7):2826-7.
- Asberg A, Jardine AG, Bignamini AA, Rollag H, et al; VICTOR Study Group. Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients. Am J Transplant. 2010;10(8):1881-8
- Andrassy J, Hoffmann VS, Rentsch M, et al. Is cytomegalovirus prophylaxis dispensable in patients receiving an mTOR inhibitor-based immunosuppression? A systematic review and meta-analysis. Transplantation. 2012;94:1208–1217.
- Su L, Tam N, Deng R, et al. Everolimus-based calcineurin-inhibitor sparing regimens for kidney transplant recipients: a systematic review and meta-analysis. Int Urol Nephrol. 2014;46:2035–2044.
- Radtke J, Dietze N, Spetzler VN, et al. Fewer cytomegalovirus complications after kidney transplantation by de novo use of mTOR inhibitors in comparison to mycophenolic acid. Transpl Infect Dis. 2016;18:79–88.
- Cervera C, Cofan F, Hernandez C, et al. Effect of mammalian target of rapamycin inhibitors on cytomegalovirus infection in kidney transplant recipients receiving polyclonal antilymphocyte globulins: a propensity score-matching analysis. Transpl Int. 2016;29:1216–1225.
7 Information, education and support
7.3 Offer balanced and accurate information to children, adults and young people undergoing solid organ transplantation about:
- The risks of CMV infection and disease according to donor / recipient CMV serostatus and nature of immunosuppression planned or received
- Monitoring and treatment options, including prophylaxis
7.4 Ensure that healthcare professionals offering information have specialist knowledge about CMV infection and disease and their treatment, and the skills to support shared decision-making (for example, presenting information in a form suitable for developmental stage)
Rationale
The committee used their own experience to derive these recommendations, noting the importance of the need to provide information to allow adequate time for decisions. Recommendations on enabling patients to actively participate in their care can be found in NICE guidance on patient experience in adult NHS services [1] and shared decision making [2].
References
- NICE Clinical guideline [CG138]: Patient experience in adult NHS services: improving the experience of care for people using adult NHS services. updated: 2021.
- NICE guideline [NG197]: Shared decision making. 2021
Information for the public
What is CMV?
CMV or cytomegalovirus is common and is related to the virus that causes cold sores, glandular fever and chicken pox. Most people have had CMV by the time they become adults. Once CMV infects someone it remains in their body for life. Infection is confirmed by the presence of CMV antibodies in the blood.
CMV causes mild, if any, symptoms in people with healthy immune systems, but it can cause severe illness following a solid-organ transplant, such as a kidney, pancreas, liver, heart or lung transplant. The risk of CMV is highest during the first year after your transplant because this when your immune system is weakened by high doses of anti-rejection medicines. It is possible, however, to develop CMV at any time after your transplant.
Can CMV be prevented after a transplant?
You will be tested for CMV antibodies in your blood, most commonly when you are added to the transplant waiting list and at the time of your transplant. If you, or the organ donor has CMV antibodies, this will not prevent you from having a transplant. It tells your doctors that you may need treatment with an antiviral medicine to prevent CMV after your transplant operation. Recommended preventive treatment is with a tablet called valganciclovir for at least three months.
How is CMV diagnosed after a transplant?
CMV after your transplant is diagnosed by a blood test. Some people have CMV after their transplant but without symptoms. If you have symptoms, they may include feeling or being sick (nausea and vomiting), diarrhoea, fever, muscle aches, fatigue, night sweats and generally feeling unwell. It is very important to get in touch with your transplant unit if you develop any of these symptoms.
How is CMV treated after a transplant?
You will be treated with antiviral medicine, usually valganciclovir, to help your body fight CMV. The antiviral medicine may be given as tablets or an intravenous infusion into a vein in hospital. You will need to have regular blood tests to check that CMV has cleared from your blood.
In some cases, your doctors may need to reduce the dose of your anti-rejection medicines to help your body fight CMV. If this happens, you will be monitored carefully for any signs of rejection of your transplant. The dose of your anti-rejection medicines will be reassessed after blood tests confirm that CMV has cleared from your body.
Will CMV affect my transplant?
In most cases treatment with antiviral medicines will get rid of CMV before it causes any serious problems. However, sometimes CMV can spread to other organ systems in your body, including your transplanted organ. This can be very serious if it is not treated, which is why it is very important to get in touch with your transplant unit if you are worried about any symptoms after your transplant.